Critically ill patients with sepsis require a multidisciplinary approach, as this situation implies multiorgan distress, with most of the bodily biochemical and cellular systems being affected by the condition. Moreover, sepsis is characterized by a multitude of biochemical interactions and by dynamic changes of the immune system. At the moment, there is a gap in our understanding of the cellular, genetic, and molecular mechanisms involved in sepsis. One of the systems intensely studied in recent years is the endocannabinoid signaling pathway, as light was shed over a series of important interactions of cannabinoid receptors with biochemical pathways, specifically for sepsis. Furthermore, a series of important implications on inflammation and the immune system that are induced by the activity of cannabinoid receptors stimulated by the delta-9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) have been noticed. One of the most important is their ability to reduce the biosynthesis of pro-inflammatory mediators and the modulation of immune mechanisms. Different studies have reported that cannabinoids can reduce oxidative stress at mitochondrial and cellular levels. In detail, the entry shows the important mechanisms modulated by the endocannabinoid signaling pathway, as well as of the molecular and cellular links it has with sepsis. At the same time, the possible implications of cannabinoids in the most important biological pathways involved in sepsis, such as inflammation, redox activity, immune system, and epigenetic expression will be presented.
- sepsis
- endocannabinoid system
- cannabinoids
- cannabis sativa
1. Biochemical Aspects of Cannabinoids
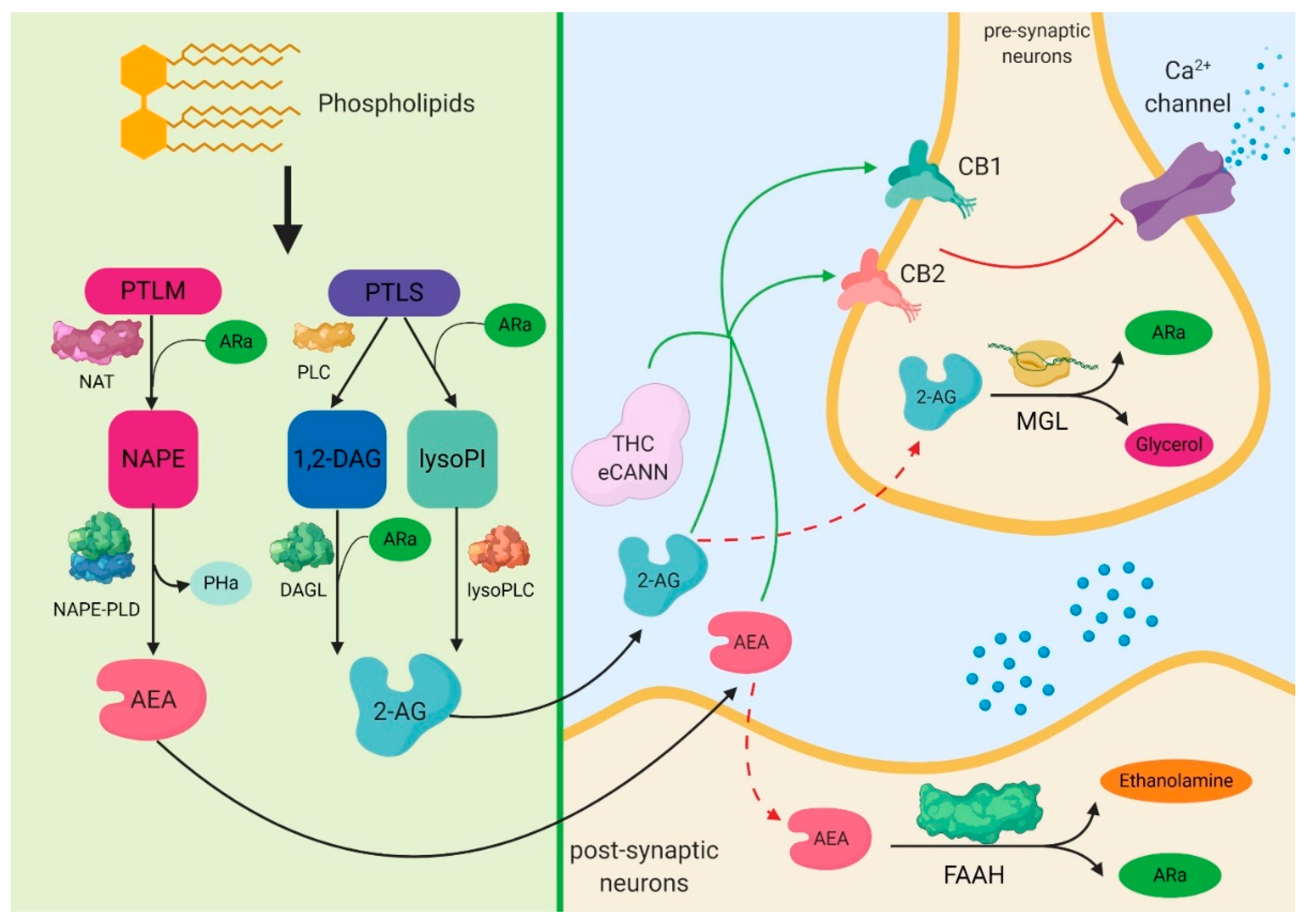
2. The Expression of Cannabinoid Signaling System in Sepsis
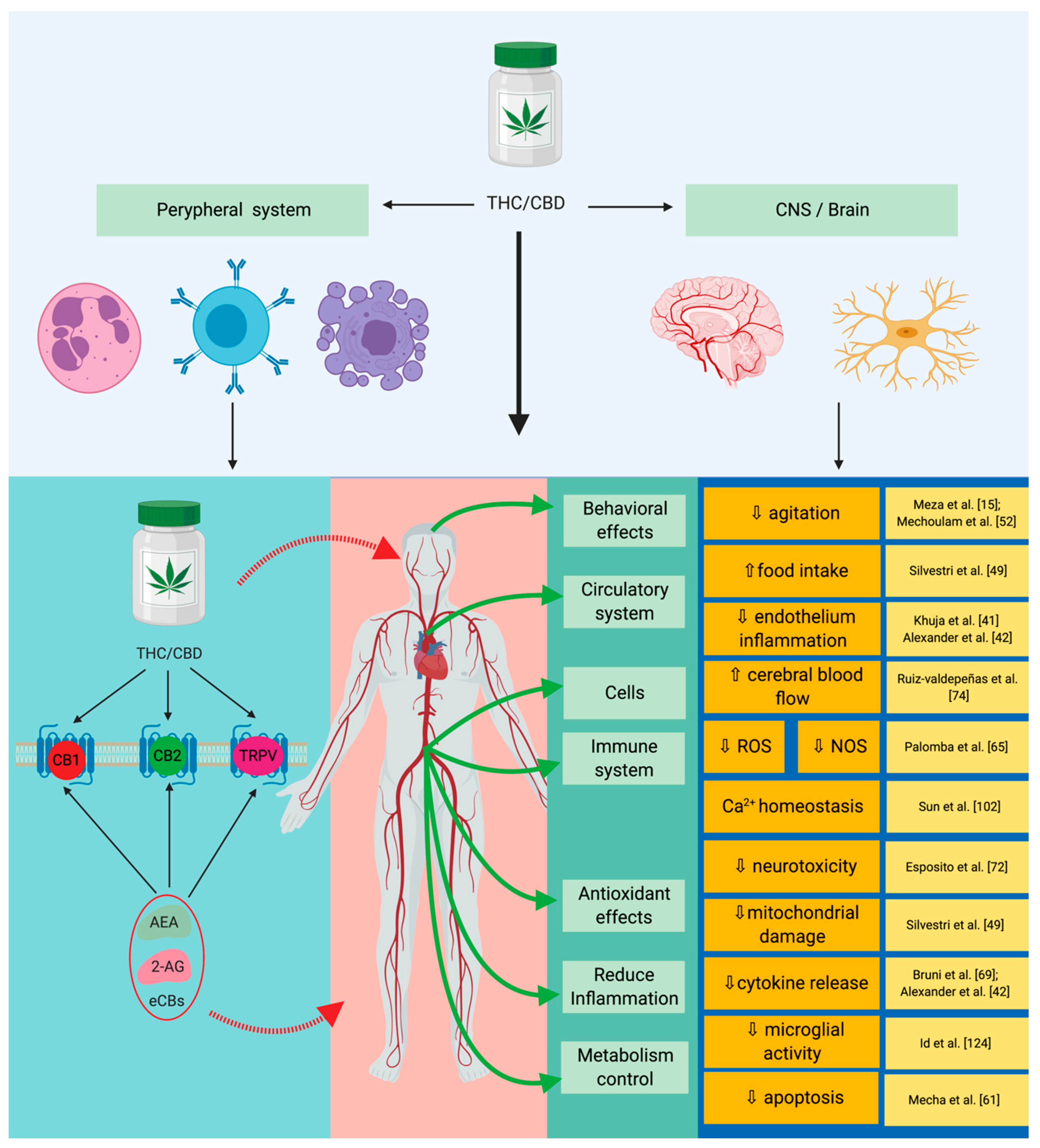
| Disorder | Observations | Reference |
|---|---|---|
| Pain | CB1 receptor agonists have a nociceptive action on the interneurons in the spinal cord, CB2 acts directly on reducing inflammation, and the CB2 receptor was shown to have an increased immunomodulatory response. | [25] |
| Cancer | The following effects have been reported: Anti-inflammatory, anti-proliferative, pro-apoptotic, anti-invasive, and anti-metastatic. | [12][13][26] |
| Hepatic metabolism | Directly acts on the modulation of the hepatic metabolism through gluconeogenesis and lipogenesis, and the CB2 receptor has a protective action on the phenomenon induced by ischemia reperfusion injury. | [9][11][27][28][29] |
| Gastrointestinal system | CB1 and CB2 receptors inhibit the pro-inflammatory and pro-oxidative activities specifically for the colon. | [11][27][30] |
| Cardiovascular system | CB2 receptor reduces inflammation specifically related to atheromatous plaques and reduces thrombosis risk; CB1 activates AMP-activated protein kinase (AMPK), reduces insulin resistance, and mimics all of the effects that encompass ischemia-reperfusion injury (IR). | [1][30][31][32] |
| Immune system/inflammation response | Reduces iNOS activity, reduces IL-6 expression; reduces TNF-α and IL-1β expression; reduces specific inflammation of ARDS/ALI; modulates and reduces the activity of TNF-α and COX-2 in the context of LPS-induced inflammation; inhibits neutrophil chemotaxis; and modulates the expression of IFN-γ, leading to the decrease of IL-2 expression. | [15][33][35][36][37][38] |
3. The Cannabinoid Signaling System and Inflammation-Linked with Sepsis
4. Cannabinoid Signaling System and Redox Activity-Linked with Sepsis
This entry is adapted from the peer-reviewed paper 10.3390/cells9020307
References
- Iseppi, R.; Brighenti, V.; Licata, M.; Lambertini, A.; Sabia, C.; Messi, P.; Pellati, F.; Benvenuti, S. Chemical characterization and evaluation of the antibacterial activity of essential oils from fibre-type cannabis sativa L. (Hemp). Molecules 2019, 24, 7–12.
- Khuja, I.; Yekhtin, Z.; Or, R.; Almogi-Hazan, O. Cannabinoids reduce inflammation but inhibit lymphocyte recovery in murine models of bone marrow transplantation. Int. J. Mol. Sci. 2019, 20, 668.
- Daniel Lafreniere, J.; Lehmann, C. Parameters of the endocannabinoid system as novel biomarkers in sepsis and septic shock. Metabolites 2017, 7, 55.
- Alexander, A.; Smith, P.F.; Rosengren, R.J. Cannabinoids in the treatment of cancer. Cancer Lett. 2009, 285, 6–12.
- Chiurchiu, V.; Leuti, A.; Cencioni, M.T.; Albanese, M.; De Bardi, M.; Bisogno, T.; Centonze, D.; Battistini, L.; Maccarrone, M. Modulation of monocytes by bioactive lipid anandamide in multiple sclerosis involves distinct Toll-like receptors. Pharmacol. Res. 2016, 113, 313–319.
- Leishman, E.; Murphy, M.; Mackie, K.; Bradshaw, H.B. BBA—Molecular and Cell Biology of Lipids Δ9-Tetrahydrocannabinol changes the brain lipidome and transcriptome di ff erentially in the adolescent and the adult. BBA-Mol. Cell Biol. Lipids 2018, 1863, 479–492.
- Chiurchiu, V.; Cencioni, M.T.; Bisicchia, E.; De Bardi, M.; Gasperini, C.; Borsellino, G.; Centonze, D.; Battistini, L.; Maccarrone, M. Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann. Neurol. 2013, 73, 626–636.
- Matthews, A.T.; Ross, M.K. Oxyradical Stress, Endocannabinoids, and Atherosclerosis. Toxics 2015, 3, 481–498.
- Chanda, D.; Kim, D.-K.; Li, T.; Kim, Y.-H.; Koo, S.-H.; Lee, C.-H.; Chiang, J.Y.L.; Choi, H.-S. Cannabinoid receptor type 1 (CB1R) signaling regulates hepatic gluconeogenesis via induction of endoplasmic reticulum-bound transcription factor cAMP-responsive element-binding protein H (CREBH) in primary hepatocytes. J. Biol. Chem. 2011, 286, 27971–27979.
- Bisogno, T.; Ligresti, A.; Di Marzo, V. The endocannabinoid signalling system: Biochemical aspects. Pharmacol. Biochem. Behav. 2005, 81, 224–238.
- Silvestri, C.; Di Marzo, V. The Endocannabinoid System in Energy Homeostasis and the Etiopathology of Metabolic Disorders. Cell Metab. 2013, 17, 475–490.
- Maione, S.; Costa, B.; Di Marzo, V. Endocannabinoids: A unique opportunity to develop multitarget analgesics. PAIN 2013, 154, S87–S93.
- Di Marzo, V. Targeting the endocannabinoid system: To enhance or reduce? Nat. Rev. Drug Discov. 2008, 7, 438–455.
- Mechoulam, R.; Parker, L.A. The Endocannabinoid System and the Brain. Annu. Rev. Psychol. 2013, 64, 21–47.
- McHugh, D.; Tanner, C.; Mechoulam, R.; Pertwee, R.G.; Ross, R.A. Inhibition of Human Neutrophil Chemotaxis by Endogenous Cannabinoids and Phytocannabinoids: Evidence for a Site Distinct from CB1 and CB2. Mol. Pharmacol. 2008, 73, 441–450.
- Tabarkiewicz, J. Endocannabinoid system as a regulator of tumor cell malignancy—Biological pathways and clinical significance. Onco Targets Ther. 2016, 9, 4323–4336.
- Gardner, E.L. Endocannabinoid signaling system and brain reward: Emphasis on dopamine. Pharmacol. Biochem. Behav. 2005, 81, 263–284.
- Watkins, B.A.; Hutchins, H.; Li, Y.; Seifert, M.F. The endocannabinoid signaling system: A marriage of PUFA and musculoskeletal health. J. Nutr. Biochem. 2010, 21, 1141–1152.
- Dogjani, A.; Zatriqi, S.; Uranues, S.; Latifi, R. Biology-based nutritional support of critically ill and injured patients. Eur. Surg. 2011, 43, 7–12.
- Vallejo, K.P.; Martínez, C.M.; Adames, A.A.M.; Fuchs-tarlovsky, V.; Carlos, G.; Nogales, C.; Enrique, R.; Paz, R.; Perman, M.I.; Isabel, M.; et al. Current clinical nutrition practices in critically ill patients in Latin America: A multinational observational study. Crit. Care 2017, 21, 227.
- Maday, K.R. Energy Estimation in the Critically Ill: A Literature Review. Int. J. Clin. Med. 2013, 1, 39–43.
- Rogobete, A.F.; Sandesc, D.; Papurica, M.; Stoicescu, E.R.; Popovici, S.E.; Bratu, L.M.; Vernic, C.; Sas, A.M.; Stan, A.T.; Bedreag, O.H. The influence of metabolic imbalances and oxidative stress on the outcome of critically ill polytrauma patients: A review. Burn. Trauma 2017, 5, 8.
- Mecha, M.; Torrao, A.S.; Mestre, L.; Carrillo-salinas, F.J.; Mechoulam, R.; Guaza, C. Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Discov. 2012, 3, e331.
- Simon, L.; Song, K.; Stouwe, C.V.; Hollenbach, A.; Amedee, A.; Mohan, M.; Winsauer, P.; Molina, P. Δ9-Tetrahydrocannabinol (Δ9-THC) Promotes Neuroimmune-Modulatory MicroRNA Profile in Striatum of Simian Immunodeficiency Virus (SIV)-Infected Macaques. J. Neuroimmune Pharmacol. 2016, 11, 192–213.
- Mechoulam, R.; Peters, M.; Murillo-Rodriguez, E.; Hanus, L.O. Cannabidiol--recent advances. Chem. Biodivers. 2007, 4, 1678–1692.
- Brown, I.; Cascio, M.G.; Rotondo, D.; Pertwee, R.G.; Heys, S.D.; Wahle, K.W.J. Cannabinoids and omega-3/6 endocannabinoids as cell death and anticancer modulators. Prog. Lipid Res. 2013, 52, 80–109.
- Palomba, L.; Silvestri, C.; Imperatore, R.; Morello, G.; Piscitelli, F.; Martella, A.; Cristino, L.; Marzo, V. Di Negative Regulation of Leptin-induced Reactive Oxygen Species (ROS) Formation by Cannabinoid CB 1 Receptor Activation in Hypothalamic Neurons. J. Biol. Chem. 2015, 290, 13669–13677.
- Lau, F.C.; Bagchi, M.; Sen, C.; Roy, S.; Bagchi, D. Nutrigenomic Analysis of Diet-Gene Interactions on Functional Supplements for Weight Management. Curr. Genom. 2008, 9, 239–251.
- Endocannabinoids, S.C.; Mukhopadhyay, P.; Horiguchi, N.; Jeong, W.; Osei-hyiaman, D.; Park, O.; Liu, J.; Harvey-white, J.; Marsicano, G.; Lutz, B.; et al. Paracrine Activation of Hepatic CB1 Receptors Mediates Alcoholic Fatty Liver. Cell. Metab. 2008, 7, 227–235.
- Prester, L.; Mikoli, A.; Juri, A.; Fuchs, N.; Neuberg, M.; Luci, A.; Br, I. Chemico-Biological Interactions Effects of Δ9-tetrahydrocannabinol on irinotecan-induced clinical effects in rats. Chem.-Biol. Interact. 2018, 294, 128–134.
- Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid delivery systems for pain and inflammation treatment. Molecules 2018, 23, 2478.
- Ladak, N.; Beishon, L.; Thompson, J.P.; Lambert, D.G. Trends in Anaesthesia and Critical Care Cannabinoids and sepsis. Trends Anaesth. Crit. Care 2011, 1, 191–198.
- Malfait, A.M.; Gallily, R.; Sumariwalla, P.F.; Malik, A.S.; Andreakos, E.; Mechoulam, R.; Feldmann, M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9561–9566.
- Meza, A.; Lehmann, C. Betacaryophyllene—A phytocannabinoid as potential therapeutic modality for human sepsis? Med. Hypotheses 2018, 110, 68–70.
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; Iuvone, T.; Steardo, L.; De Filippis, D.; Cipriano, M.; Carratu, M.R. Cannabidiol Reduces Aβ-Induced Neuroinflammation and Promotes Hippocampal Neurogenesis through PPARγ Involvement. PLoS ONE 2011, 6, e28668.
- Ribeiro, A.M.; Homsi, C.; Ferreira, J.; Mateus-vasconcelos, E.C.L.; Moroni, R.M.; Maria, L.; Brito, O.; Gustavo, L.; Brito, O. Case Report Physical Therapy in the Management of Pelvic Floor Muscles Hypertonia in a Woman with Hereditary Spastic Paraplegia. Case Rep. Obstet. Gynecol. 2014, 2014, 306028.
- Ruiz-valdepeñas, L.; Martínez-orgado, J.A.; Benito, C.; Millán, Á.; Tolón, R.M. Cannabidiol reduces lipopolysaccharide-induced vascular changes and inflammation in the mouse brain: An intravital microscopy study Cannabidiol reduces lipopolysaccharide-induced vascular changes and inflammation in the mouse brain: An intravital micros. J. Neuroinflamm. 2011, 8, 5.
- Chen, W.; Kaplan, B.L.F.; Pike, S.T.; Topper, L.A.; Lichorobiec, N.R.; Simmons, S.O.; Ramabhadran, R.; Kaminski, N.E. Magnitude of stimulation dictates the cannabinoid-mediated differential T cell response to HIVgp120. J. Leukoc. Biol. 2012, 92, 1093–1102.
- Cassol, O.J., Jr.; Comim, C.M.; Silva, B.R.; Hermani, F.V.; Constantino, L.S.; Felisberto, F.; Petronilho, F.; Hallak, J.E.C.; De Martinis, B.S.; Zuardi, A.W.; et al. Treatment with cannabidiol reverses oxidative stress parameters, cognitive impairment and mortality in rats submitted to sepsis by cecal ligation and puncture. Brain Res. 2010, 1348, 128–138.
- Vuolo, F.; Petronilho, F.; Sonai, B.; Ritter, C.; Hallak, J.E.C.; Zuardi, A.W.; Crippa, J.A.; Dal-pizzol, F. Evaluation of Serum Cytokines Levels and the Role of Cannabidiol Treatment in Animal Model of Asthma. Mediat. Inflamm. 2015, 2015, 538670.
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419.
- Hernández-cervantes, R.; Méndez-díaz, M.; Prospéro-garcía, Ó. Immunoregulatory Role of Cannabinoids during Infectious Disease. Neuroimmunomodulation 2017, 24, 183–199.
- Wasim, K.; Haq, I.; Ashraf, M. Antimicrobial studies of the leaf of cannabis sativa L. Pak. J. Pharm. Sci. 1995, 8, 29–38.
- Elphick, M.R. BfCBR: A cannabinoid receptor ortholog in the cephalochordate Branchiostoma floridae (Amphioxus). Gene 2007, 399, 65–71.
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430.
- Bass, R.; Engelhard, D.; Trembovler, V.; Shohami, E. A novel nonpsychotropic cannabinoid, HU-211, in the treatment of experimental pneumococcal meningitis. J. Infect. Dis. 1996, 173, 735–738.
- Chakraborty, S.; Bisoi, S.; Chattopadhyay, D.; Mishra, R. A Study on Demographic and Clinical Profile of Burn Patients in an Apex Institute of West Bengal. Indian J. Public Health. 2010, 54, 92–94.
- Bellocchio, L.; Lafenetre, P.; Cannich, A.; Cota, D.; Puente, N.; Grandes, P.; Chaouloff, F.; Piazza, P.V.; Marsicano, G. Bimodal control of stimulated food intake by the endocannabinoid system. Nat. Neurosci. 2010, 13, 281–283.
- Sardinha, J.; Kelly, M.E.M.; Zhou, J.; Lehmann, C. Experimental Cannabinoid 2 Receptor-Mediated Immune Modulation in Sepsis. Mediat. Inflamm. 2014, 2014, 978678.
- Wang, L.-L.; Zhao, R.; Li, J.-Y.; Li, S.-S.; Liu, M.; Wang, M.; Zhang, M.-Z.; Dong, W.-W.; Jiang, S.-K.; Zhang, M.; et al. Pharmacological activation of cannabinoid 2 receptor attenuates inflammation, fibrogenesis, and promotes re-epithelialization during skin wound healing. Eur. J. Pharmacol. 2016, 786, 128–136.
- Eisenstein, T.K.; Meissler, J.J. Effects of Cannabinoids on T-cell Function and Resistance to Infection. J. Neuroimmune Pharmacol. 2015, 10, 204–216.
- Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Mariano-Souza, D.P.; Quinteiro-Filho, W.M.; Akamine, A.T.; Almeida, V.I.; Quevedo, J.; Dal-Pizzol, F.; et al. Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: Role for the adenosine A2A receptor. Eur. J. Pharmacol. 2012, 678, 78–85.
- Obeid, R.; Herrmann, W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 2006, 580, 2994–3005.
- Naviaux, R.K. Mitochondrion Metabolic features of the cell danger response. MITOCH 2014, 16, 7–17.
- Horhat, F.G.; Rogobete, A.F.; Papurica, M.; Sandesc, D.; Tanasescu, S.; Dumitrascu, V.; Licker, M.; Nitu, R.; Cradigati, C.A.; Sarandan, M.; et al. The Use of Lipid Peroxidation Expression as a Biomarker for the Molecular Damage in the Critically Ill Polytrauma Patient. Clin. Lab. 2016, 62, 1601–1607.
- Melo, A.C.; Valença, S.S.; Gitirana, L.B.; Santos, J.C.; Ribeiro, L.M.; Machado, M.N.; Magalhães, C.B.; Zin, W.A.; Porto, L.C. Redox markers and inflammation are differentially affected by atorvastatin, pravastatin or simvastatin administered before endotoxin-induced acute lung injury. Int. Immunopharmacol. 2013, 17, 57–64.
- Moise, A. Vitamin D in Critically Ill Patients - From Molecular Damage Interactions to Clinical Outcomes Benefits. When, Why, How? Central. European. J. Clin. Res. 2018, 1, 59–66.
- Sandesc, M.; Rogobete, A.F.; Bedreag, O.H.; Dinu, A.; Papurica, M.; Cradigati, C.A.; Sarandan, M.; Popovici, S.E.; Bratu, L.M.; Bratu, T.; et al. Analysis of oxidative stress-related markers in critically ill polytrauma patients: An observational prospective single-center study. Bosn. J. Basic Med. Sci. 2018, 18, 191–197.
- Bedreag, O.H.; Rogobete, A.F.; Sandesc, D.; Cradigati, C.A.; Sarandan, M.; Popovici, S.E.; Dumache, R.; Horhat, F.G.; Vernic, C.; Sima, L.V.; et al. Modulation of the Redox Expression and Inflammation Response in the Critically Ill Polytrauma Patient with Thoracic Injury. Statistical Correlations between Antioxidant Therapy and Clinical Aspects. A Retrospective Single Center Study. Clin. Lab. 2016, 62, 1747–1759.
- Hu, Y.; Deng, H.; Xu, S.; Zhang, J. MicroRNAs Regulate Mitochondrial Function in Cerebral Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2015, 16, 24895–24917.
- Fredriksson, K.; Tjäder, I.; Keller, P.; Petrovic, N.; Ahlman, B.; Schéele, C.; Wernerman, J.; Timmons, J.A.; Rooyackers, O. Dysregulation of mitochondrial dynamics and the muscle transcriptome in ICU patients suffering from sepsis induced multiple organ failure. PLoS ONE 2008, 3, e3686.
- Sun, S.; Sursal, T.; Adibnia, Y.; Zhao, C.; Zheng, Y.; Li, H.; Otterbein, L.E.; Hauser, C.J.; Itagaki, K. Mitochondrial DAMPs Increase Endothelial Permeability through Neutrophil Dependent and Independent Pathways. PLoS ONE 2013, 8, e59989.
- Ross, J.A.; Tolar, J.; Spector, L.G.; DeFor, T.; Lund, T.C.; Weisdorf, D.J.; Langer, E.; Hooten, A.J.; Thyagarajan, B.; Gleason, M.K.; et al. An exploratory analysis of mitochondrial haplotypes and allogeneic hematopoietic cell transplantation outcomes. Biol. Blood Marrow Transplant. 2015, 21, 81–88.
- Yao, X.; Carlson, D.; Sun, Y.; Ma, L.; Wolf, S.E.; Minei, J.P.; Zang, Q.S. Mitochondrial ROS induces cardiac inflammation via a pathway through mtDNA damage in a pneumonia-related sepsis model. PLoS ONE 2015, 10, e0139416.
- Liu, X.; Chen, Z. The pathophysiological role of mitochondrial oxidative stress in lung diseases. J. Transl. Med. 2017, 15, 207.
- Sun, S.; Hu, F.; Wu, J.; Zhang, S. Redox Biology Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol. 2017, 11, 577–585.
- Burstein, S. Bioorganic & Medicinal Chemistry Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385.
- Carrier, E.J.; Auchampach, J.A.; Hillard, C.J. Inhibition of an equilibrative nucleoside transporter by cannabidiol: A mechanism of cannabinoid immunosuppression. Proc. Natl. Acad. Sci. USA 2006, 103, 7895–7900.
- Castillo, R.L.; Loza, R.C. Pathophysiological Approaches of Acute Respiratory Distress syndrome: Novel Bases for Study of Lung Injury. Open Respir. Med. J. 2015, 9, 83–91.
- Hebert-Chatelain, E.; Desprez, T.; Serrat, R.; Bellocchio, L.; Soria-Gomez, E.; Busquets-Garcia, A.; Pagano Zottola, A.C.; Delamarre, A.; Cannich, A.; Vincent, P.; et al. A cannabinoid link between mitochondria and memory. Nature 2016, 539, 555.
- Ryan, D.; Drysdale, A.J.; Lafourcade, C.; Pertwee, R.G.; Platt, B. Cannabidiol Targets Mitochondria to Regulate Intracellular Ca+2 Levels. J. Neurosci. 2009, 29, 2053–2063.
