Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
MXenes are synthesized from ‘MAX’ phases by the selective etching of ‘A’ layers. The MAX phases are conductive 2D layers of transition metal carbides/nitrides interconnected by the ‘A’ element with strong ionic, metallic, and covalent bonds.
- MXene
- 2D materials
- biomedical applications
1. Introduction
Research on 2D materials can be traced back to the pioneering work of Langmuir on elemental monolayers in the 1930s [1]. The long-forgotten research area underwent a reawakening with the discovery of graphene, the first two-dimensional atomic crystal, in 2004 [2,3], and its profound success thereafter. Since then, 2D materials such as hexagonal boron nitride, transition metal dichalcogenides, phosphorenes, etc. have been discovered and explored for promising applications [4]. MXenes (pronounced ‘maxenes’) emerged as an elegant member of the above category and soon proved to be versatile enough to revolutionize many aspects of human life by replacing some of the commonly used 2D materials to become the next disruptive technology. MXenes are synthesized from ‘MAX’ phases by the selective etching of ‘A’ layers. The MAX phases are conductive 2D layers of transition metal carbides/nitrides interconnected by the ‘A’ element with strong ionic, metallic, and covalent bonds [5]. As shown in Figure 1, a typical MXene 2D flake is formed by transition elements such as Sc, Ti, V, Cr, Mn, Fe, Y, Zr, Nb, Mo, Hf, Ta, and W interleaved by carbon or nitrogen with the general formula Mn+1XnTx, where Tx represents surface functionalities such as F, Cl, O, and OH [6,7,8]. The history of MXenes begins in the year 2011 with the synthesis of 2D-layered Ti3C2Tx from the exfoliation of Ti3AlC2 MAX phase by Gogotsi‘s group [9]. The initial synthesis approach was conceptualized based on the weak Ti-Al metallic bond. This enabled the easy removal of Al atoms from the Ti3AlC2 MAX phase, such as AlF3, which was later removed by simple washing and resulted in a multilayered, accordion-like structure. This etching process was widely explored for the synthesis of different MXenes, and parameters such as etching time and HF concentration were optimized [10,11]. Owing to the high risk in handling and the corrosive nature of HF, several lower-risk alternative approaches have been conceptualized. Some of these approaches involved chemicals or combinations of chemicals such as NH4HF2 [12], HCl/FeF3 [13], HCl/LiF [14], HCl/NaF [15], HCl/KF [16], HCl/NH4F/KF [17], and HCl/NH4F [18], which can act as an in situ source of fluoride ions and improve the safety in operation to a large extent. Nowadays, fluorine-free synthesis approaches are gaining momentum as a new, safer gateway to MXene synthesis, and many innovative top-down synthesis routes, such as electrochemical etching [19,20], thermally assisted electrochemical approaches [21], hydrothermal treatments in NaOH [22] and KOH solutions [23], element replacement by reaction with Lewis acid molten salts [24], salt-templated approaches [25], etc., have been introduced. Moreover, bottom-up synthesis by chemical vapor deposition (CVD), plasma-enhanced pulsed laser deposition (PEPLD), and template methods [26,27] were also reported for the synthesis of MXenes. Because of their 2D planar structure, hydrophilicity, endless and flexible functionalization possibilities, strong absorption in the near-infrared (NIR) region, and exceptional properties, biomedical applications emerged as one of the most promising application fields of MXenes (Scheme 1). MXenes are found to be suitable candidates for applications including anticancer and drug delivery, antimicrobial, photothermal therapy, biosensors, and tissue engineering. However, even with intensive research efforts on MXene, the outstanding properties of these materials alone still cannot meet all the requirements of various biomedical applications. To endow new functions and to improve the performance, MXenes were functionalized, and their surfaces modified. Recently, functional modification of MXenes and the combination of MXenes with 3D [28], 2D [29], 1D [30], 0D [31], and polymer materials [32] with covalent and non-covalent modifications opened a new horizon for the functional requirements of biomedical applications. MXenes were modified with heteroatoms such as sulfur [33], phosphorous [34], and nitrogen [35] to produce functional MXenes. Apart from this, MXenes with enhanced properties were synthesized by doping with boron [36], platinum [37], niobium [38], silicon and germanium [39], vanadium [40], and alkali and alkaline earth metal cations [41]. As an ideal biomaterial for biomedical applications, MXenes, and their composites could be engineered with different physical, mechanical, or chemical properties [42], and must be compatible with the physiological environment with reliable mechanical strength, degradability, and the ability to overcome biological rejection [43]. Even though they have been less explored, several MXenes and their composites have proven to be biocompatible and non-toxic to living organisms [44], and MXenes such as niobium carbide have proven to be biodegradable in mice [45], thereby proving promising for in vivo applications.
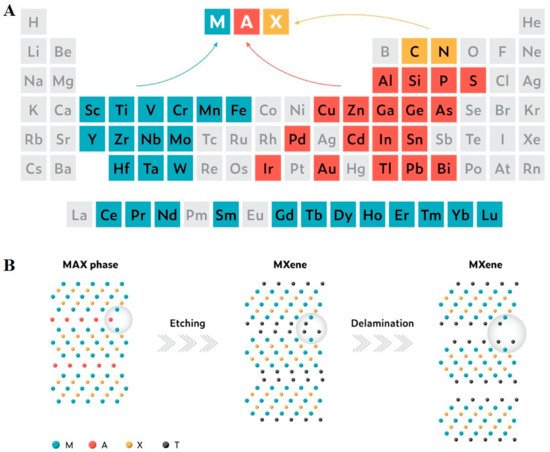
Figure 1. (A) The periodic table highlighting the ‘M’, ‘A’, and ‘X’ elements of known MAX phases. (B) Schematic illustration of the synthesis of MXenes from MAX phases. Reprinted with permission from Ref. [46]. Copyright © 2022 Wiley.
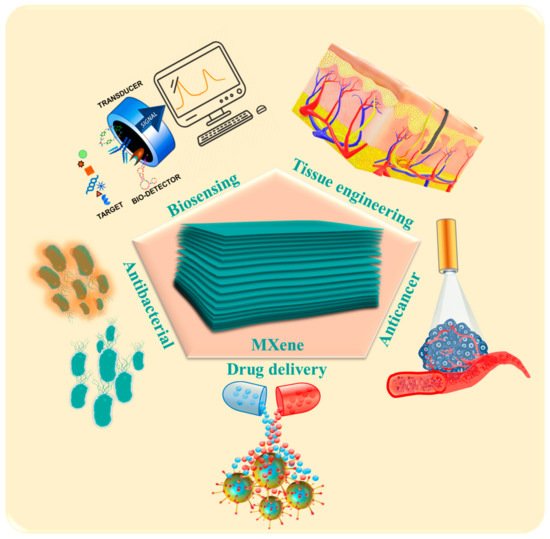
Scheme 1. Biomedical applications of MXenes.
2. Drug Delivery Applications
The unique 2D planar structure and physicochemical properties of MXenes make them favorable for precision drug delivery applications [47]. Ti3C2Tx, a prominent member of the MXene family, is renowned for its drug delivery applications (Table 1) because of its ultrathin planar nanostructure, excellent photothermal conversion capability, excellent near-infrared (NIR) responsiveness, and the chemically tunable nature of the surface functionalities [48,49,50]. An ideal drug delivery system requires controllability and sufficient drug-loading capability so that the drug-carrying nanovehicles continuously stay in the required body part. However, Ti3C2Tx-based nanovehicles lack sufficient controllability and suffer low drug-loading capability, so that, with blood circulation, the drug-carrying nanovehicles are continuously removed from the body site of application and cause inevitable damage to normal tissue [51]. Similar to other inorganic 2D materials, MXene-based nanoplatforms suffer stability in physiological conditions [52], which may affect the controllable release of the drug for cancer therapy. Therefore, fabricating a smart MXene-based nanoplatform for drug delivery remained a challenge. Controllability of a MXene-based drug carrier may be improved by adding magnetic nanomaterials so that the drug carrier could be controlled and confined to the target cells by the application of an external magnetic field. The drug, then, will be released by an endogenous or exogenous stimulation [53].
3. Antimicrobial Applications
Microbial growth is considered a serious health concern. Among various 2D materials, MXenes (particularly Ti3C2Tx) have emerged as a promising candidate, showing antimicrobial activity even higher than graphene oxide [84]. MXenes have shown enhanced antimicrobial activity because of the enhanced cell membrane permeability, membrane rupture, DNA destruction because of the sharp edges, hydrophilicity, and hydrogen bonding with the cell membrane lipopolysaccharide molecules [85]. MXenes and their composites have shown excellent antibacterial properties against Escherichia coli, Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, and Shigella (Table 3). MXene functional groups are also reported to cause cell inactivation by preventing the intake of nutrients and thereby inhibiting the growth of bacteria [86]. The atomic structures of MXenes have been reported to have a crucial role in the antimicrobial properties of MXene [87]. Some MXenes such as Ti3C2Tx and TiVCTx are reported to possess inherent antimicrobial properties. Additionally, the transfer of reactive electrons caused by the formation of a conductive bridge over the lipid bilayer from the bacterial cell to the external environment enables ultimate cell death [86]. Among the various other factors influencing the antibacterial efficiency of MXenes, environmental conditions and the structure of bacterial cell walls play a crucial role. Environmental conditions contribute to the aging of the membrane, and surface oxidation of Ti3C2Tx in the air results in the formation of nanocrystals of anatase TiO2 [88]. The TiO2 catalyzed free radical formation enhances the antibacterial property of Ti3C2Tx by stimulating oxidative stress on bacterial cell walls [88]. Since peptidoglycan thickness varies in gram-negative and gram-positive bacteria (peptidoglycan is thin in E. coli [89] and thick in B. subtilis [90]), a corresponding difference is observed in the resistance towards the MXene. Recently, the stoichiometry of the MXenes with the same chemical composition was also reported to exert considerable influence on its antibacterial activity [87].
Table 3. Literature reports on the antimicrobial applications of MXene/Composites.
| MXene/Composite | Antimicrobial Applications | Ref. |
|---|---|---|
| Ti3C2Tx | Antibacterial activity against E. coli and B. subtilis with 98% viability loss within 4 h. | [84] |
| Colloidal Ti3C2Tx | Antibacterial activity against B. subtilis and E. coli. | [85] |
| Ti3C2Tx | Antibacterial activity against E. coli. | [87] |
| Ti3C2Tx | Photocatalytic inactivation of airborne E. coli. | [93] |
| Bi2S3/Ti3C2Tx | Photoexcited antimicrobial effects on S. aureus and E. coli. | [94] |
| Ti3C2Tz/Chitosan | Antibacterial activity against E. coli and S. aureus. | [95] |
| Nb2CTx and Nb4C3Tx | Bactericidal property against E. coli and S. aureus. | [96] |
| Cu2O/Ti3C2Tx | Antibacterial activity against S. aureus and Pseudomonas aeruginosa. | [97] |
| Ti3C2Tx-AuNCs | Antibacterial performance on S. aureus and E. coli. | [98] |
| MoS2/Ti3C2Tx | Antibacterial activity against E. coli and B. subtilis. | [99] |
| Ti3C2Tx-Laden bacteriophage | Antibacterial activity against Shigella. | [100] |
| Ag/Ti3C2Tx | Inhibitory activity against E. coli and S. aureus. | [101] |
| TiVCTX | Antibacterial activities against E. coli, photothermal sterilization effect on E. coli and B. subtilis. | [102] |
| CuP-sTi3C2Tx | Antibacterial activity against E. coli and S. aureus. | [103] |
| Ti3C2Tx | Size-dependent photothermal antibacterial activity against S. aureus. | [104] |
| Ti3C2Tx/PVA hydrogel | Antibacterial activity against E. coli and S. aureus. | [105] |
| V2C NSs | Antibacterial activity against E. coli, and B. subtilis. | [106] |
| BC/Chi/Ti3C2Tx/AgNWs aerogel | Antibacterial activity against E. coli and S. aureus. | [107] |
This entry is adapted from the peer-reviewed paper 10.3390/bios12070454
This entry is offline, you can click here to edit this entry!
