Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Natural killer (NK) cells play a pivotal role in cancer immunotherapy due to their innate ability to detect and kill tumorigenic cells. The decision to kill is determined by the expression of a myriad of activating and inhibitory receptors on the NK cell surface. Cell-to-cell engagement results in either self-tolerance or a cytotoxic response, governed by a fine balance between the signaling cascades downstream of the activating and inhibitory receptors.
- natural killer cells
- NK cells
- immune surveillance
- signaling
- NK cell receptors
1. Introduction
Natural Killer (NK) cells are bone marrow–derived innate lymphocytes that are found in most organs, with the largest population of NK cells residing in the blood [1]. NK cells are large granular lymphocytes that were initially defined by their ability to kill tumor cells without prior sensitization [2][3]. The role of NK cells has since been expanded to include the elimination of virally infected cells and secretion of cytokines that mediate crosstalk and regulation of other immune cells [4].
Following their discovery in the 1970s, immunologists have been fascinated by the ability of NK cells to detect and kill tumorigenic or virally-infected cells, whilst tolerating healthy self-tissue [5][6][7]. However, it wasn’t until the early 1990s that scientists started to explore the mechanisms by which NK cells distinguished “self” from “non-self,” an area of research instigated by Klas Kärre’s exposition of the “missing self” theory [8]. Karre hypothesized that NK cells could recognize loss or reduction in surface expression of major histocompatibility complex (MHC) class I proteins (human leukocyte antigens (HLA) class I in humans), triggering recognition as non-self. This hypothesis was based on earlier studies by Strokus et al. [9][10] that described protection of susceptible cells with experimentally expressed MHC-I. The ‘missing-self’ theory was further validated by Karlhofer et al. [11], who showed that the murine Lymphocyte Ag 49A (Ly49A) receptor recognized and discriminated between different MHC-I molecules, with tumor cells from H2d and H2K backgrounds resistant to killing by Ly49A expressing NK cells [11][12]. Soon after, Moretta et al. [13] discovered the first human inhibitory NK receptor Killer cell immunoglobulin-like receptor 2DL1 (KIR2DL). Inhibitory receptors are now known to not only sense reduction in expression of MHC class I proteins, but also recognize non-MHC-I molecules, such as glycans and collagen, which are crucial for NK cell discrimination of self [14]. Upon engagement of cognate ligands, the various NK cell receptors send activating and inhibitory signals, which collectively determine NK cell action.
NK cells kill infected and transformed cells via a variety of mechanisms, including the delivery of lytic granules loaded with proteases and pore-forming proteins such as granzymes and perforin, release of cytokines such as tumor necrosis factor alpha (TNFα) and interferon gamma (IFNγ), upregulation of FASL and TNF-related apoptosis-inducing ligand (TRAIL) and by antibody-dependent cellular cytotoxicity (ADCC) [15][16][17][18][19]. There are multiple steps between NK cell: target cell engagement and cell killing, with receptor-ligand interactions thought to be the initiating step in the formation of an immunological synapse (IS) (Figure 1A,B) [20][21]. This is followed by recruitment of filamentous actin (F-actin) to the IS (Figure 1B) and polarization of the lytic granules and the microtubule-organizing center (MTOC) toward the IS (Figure 1C). Then, the granules dock at the synapse and are ready for the final step: granule-membrane fusion and release of the cytotoxic contents at the center of the IS (Figure 1D) [22][23]. NK cell signaling and killing is considered to be localized to the IS [24], with each NK cell thought to reach exhaustion after killing four to seven target cells [25].
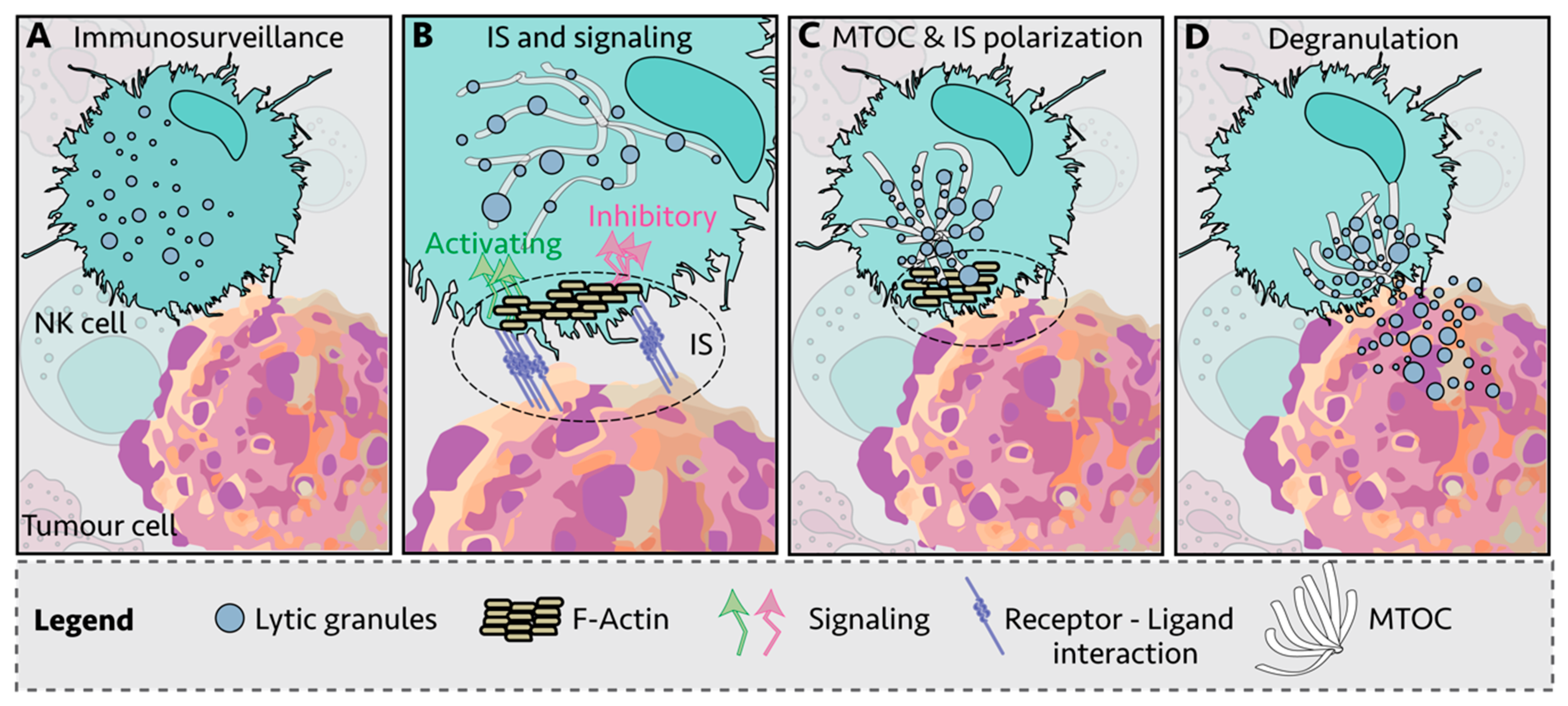
Figure 1. Immunological synapse (IS) of Natural Killer (NK) cell and target cell. (A) NK cells engage other cells via integrins and adhesion molecules, which create the immunological synapse (IS)—the subsequent process between engagement and killing or tolerance can be broken down into four steps. (B) First, filamentous actin (F-actin) is recruited to the IS. Inside-out signaling reinforces IS interactions and activating and inhibitory surface receptors cluster at the IS. (C) Second, NK lytic granules move along microtubules by dynein-dynactin motor proteins toward the microtubule-organizing center (MTOC). (D) Third, the polarized lytic granules and MTOC travel in an ATP-dependent manner through the actin mesh via myosin IIA to dock at the IS, and finally, the lytic granules fuse with the membrane and release the lytic contents into the target cells, a process also known as degranulation. The NK cell then detaches and moves on to the next target.
2. Receptor Mediated Inhibition of NK Cells: Inhibitory Receptors
NK cell self-tolerance in humans and mice is mostly mediated by inhibitory receptors that recognize either MHC-I complexes or non-MHC-I surface molecules [26]. In general, inhibitory receptors belong to receptor families comprised of both activating and inhibitory members, and signal through a cytoplasmic signaling tail containing an immunoreceptor tyrosine-based inhibitory motif (ITIM) (Table 1, Figure 2) [27]. NK killing of target cells can be viewed as an internal decision-making process using a “pros and cons” list; when activating signals outweigh inhibitory signals, the NK cell becomes cytotoxic. Interestingly, NK cells lacking inhibitory receptors are unable to become cytotoxic, thus acquiring functional maturation is also dependent on inhibitory signals. This requirement is commonly referred to as NK cell “education” [28].
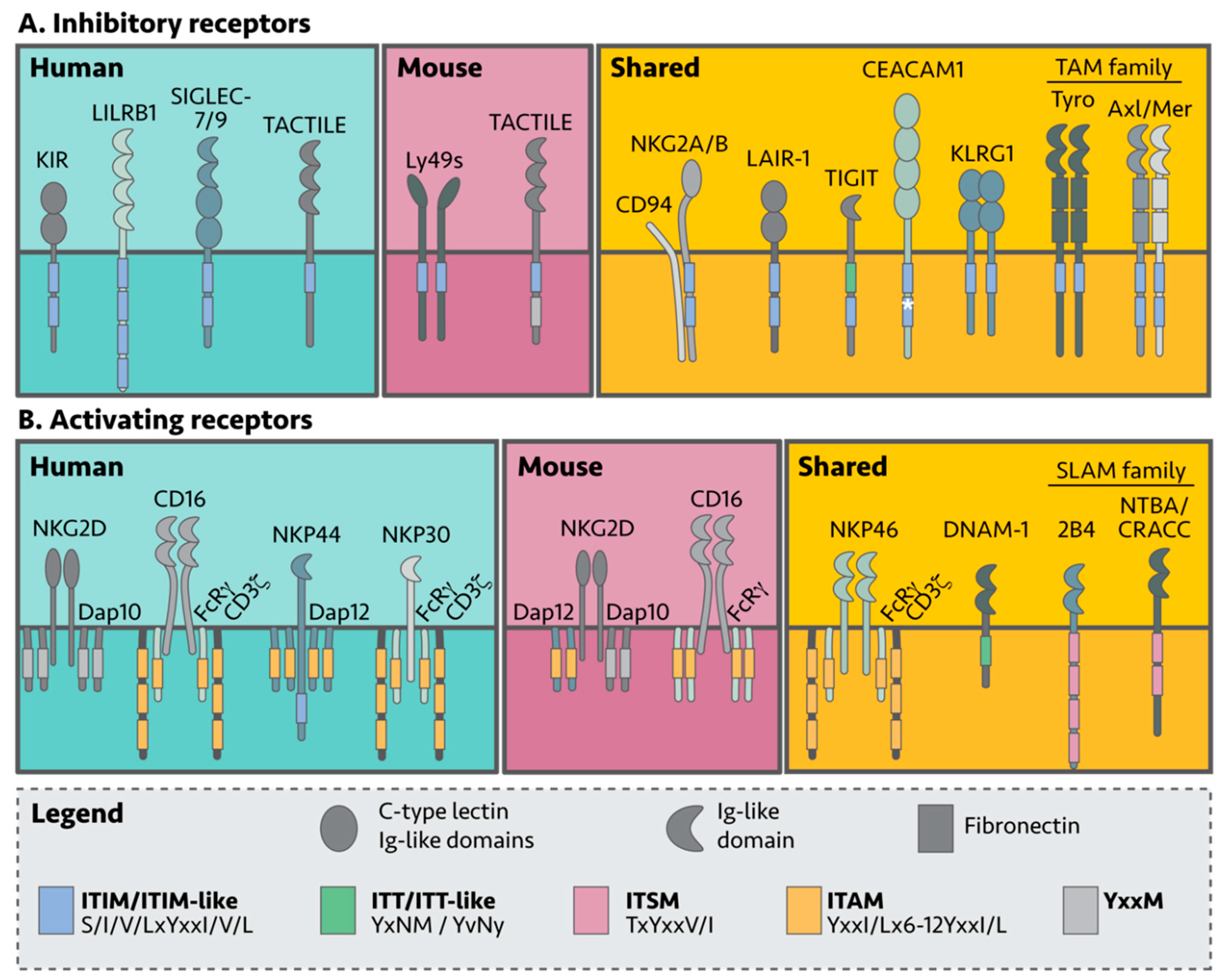
Figure 2. NK cell surface receptors involved in tumor recognition. NK cells express a myriad of inhibitory and activating receptors designed to recognize healthy or aberrant (non-healthy) cells. (A) Inhibitory receptors dampen activating NK cell signals via cytoplasmic tyrosine motifs in their cytoplasmic tails, regulating NK cell effector function. (B) In contrast, most activating receptors signal through cytoplasmic adaptor proteins. Although many of the receptors are expressed by both mouse and human NK cells (shared), some are exclusive.
Table 1. Consensus sequence of signaling motifs.
| Signaling Motifs | Consensus Sequence |
|---|---|
| ITIM | S/I/V/LxYxxI/V/L |
| ITT/ITT-Like | YxNM/YvNy |
| ITSM | TxYxxV/I |
| ITAM | YxxI/Lx6-12YxxI/L |
3. Current Therapies Harnessing the Power of Inhibitory NK Receptors
The success of current immunotherapies that block immune checkpoints, such as CTLA4 and PD1 in CD8 cells, has spurred interest in targeting other immune populations, such as NK cells. Given that blocking NK inhibitory receptors has proven beneficial in experimental tumor models, a number of blocking antibodies have been developed, with several progressing to clinical trials. Currently, KIR2D and NKG2A are the only two MHC-I-dependent receptors targeted to mimic a “missing-self” situation and enhance anti-tumor activity, with two agents that block KIR2D (Lirilumab and IPH2101) in clinical phase I or II trials. Lirilumab alone or in combination with other agents is being trialed against solid tumors (NCT03203876), hematological malignancies [29], chronic lymphocytic leukemia (NCT02557516), multiple myeloma (NCT01592370), resectable squamous cell carcinoma of the head and neck (NCT03341936), and resectable bladder cancer (NCT03532451), while IPH2101 is being trialed against acute myeloid leukemia [30]. Monalizumab is the only antibody being trialed for NKG2A, both alone and in combination with other agents for advanced gynecological solid tumors [31], advanced squamous cell carcinoma of the head and neck (NCT02643550), and resectable non-small cell lung cancer (NCT03794544).
In addition to KIR2D and NKG2A, anti-TIGIT antibodies are in multiple phase I and II clinical trials against different advanced solid tumors (NCT029133133, NCT02794571 and NCT02964013). Anti-TIGIT antibodies are also being used in combination with other therapies against solid tumors (NCT04150965, NCT03119428 and NCT04047862), notably against advanced non-small cell lung cancer (NCT03563716). These are just a handful of the clinical trials that are taking advantage of blocking inhibitory NK signaling, with no doubt many more to come.
4. Inhibitory NK Signaling
The full complement of events that transduce inhibitory receptor signals remains unclear, with much work still required to fully understand the different pathways. However, for some receptors a clearer picture of the signaling events following ligand engagement is now emerging, as well as identification of alternative signaling cascades that can lead to inhibition.
Inhibitory NK receptors signal via the different tyrosine-containing motifs: ITIM, ITT, ITSM, ITIM-like, ITT-like, or ITSM-like motifs. Upon engagement of the respective ligands, tyrosines within the signaling motifs are phosphorylated by members of the Src-family kinases (SFKs), with Lyn and Lck the most likely candidates in NK cells (Figure 3A) [32][33][34]. Tyrosine phosphorylation within the inhibitory motif enables recruitment of Src homology 2 (SH2)-containing protein tyrosine phosphatases such as SHP-1 and SHP-2, which then function to de-phosphorylate signaling intermediates and negatively regulate NK cell activity.
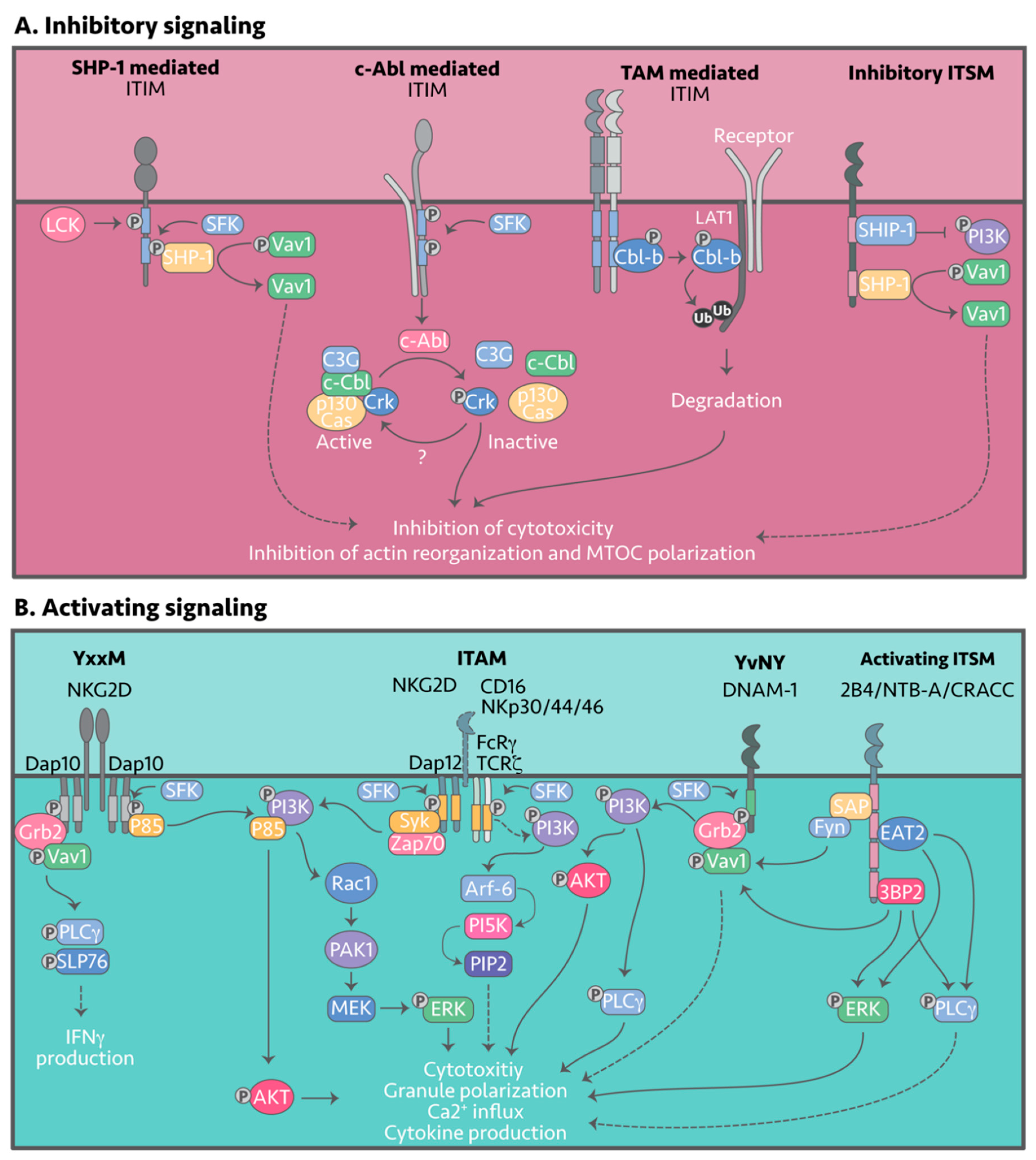
Figure 3. Inhibitory and activating NK cell signaling. NK cell effector function is collectively determined by the strongest activating or inhibitory signals. (A) Signaling downstream of inhibitory receptors is initiated by ligand engagement, followed by tyrosine phosphorylation of the signaling motif by Src-family kinases (SFK), Fyn or Lck. Once phosphorylated, there are three known inhibitory pathways: (1) Recruitment of SHP-1, SHP-2 or SHIP, which dephosphorylate Vav1; (2) Association with c-Abl kinase, which phosphorylates Crk disassociating it from its active complex and; (3) Phosphorylation and activation of Cbl-b by the TAM receptors. Cbl-b in turn ubiquitylates activating signaling intermediates such as LAT1 for degradation. (B) Signaling downstream of activating receptors is similarly transduced by tyrosine containing motifs that are phosphorylated by SFK. Various signaling intermediates such as Grb2, VAV1, or PI3K are then recruited, which induce cytotoxicity and cytokine release.
5. Current Therapies Harnessing the Power of Activating NK Receptors
There are several ongoing clinical trials testing antibodies that enhance NK cell activation, mediate direct cell killing (ADCC) or achieve both NK cell activation and ADCC. The latter is exemplified by Elozutumab, an anti-CRACC (SLAM7) antibody currently in pre-clinical testing and phase 1–3 clinical trials for multiple myeloma (NCT01335399) [35][36][37]. Another ongoing trial in non-Hodgkin’s lymphoma is combining anti-CD123 antibody with adoptive transfer of an NK cell line engineered to express high levels of CD16 and potentiate NK responses (NCT03027128) [38]. Adoptively transferred, allogeneic CD19 CAR-NK cells were successfully used in recent phase 1 and 2 trials to treat patients with non-Hodgkin’s lymphoma or chronic lymphocytic leukemia (CLL) without significant toxicities [39]. These studies demonstrate the importance of NK cell therapies and pave the way for further clinical trials using blocking antibodies and/or CAR-NK cells expressing activating receptors [40][41][42].
This entry is adapted from the peer-reviewed paper 10.3390/cancers12040952
References
- Gregoire, C.; Chasson, L.; Luci, C.; Tomasello, E.; Geissmann, F.; Vivier, E.; Walzer, T. The trafficking of natural killer cells. Immunol. Rev. 2007, 220, 169–182.
- Ortaldo, J.R.; Wiltrout, R.H.; Reynolds, C.W. Natural killer activity: Early days, advances, and seminal observations. Crit. Rev. Oncog. 2014, 19, 1–13.
- Rosenberg, E.B.; Herberman, R.B.; Levine, P.H.; Halterman, R.H.; McCoy, J.L.; Wunderlich, J.R. Lymphocyte cytotoxicity reactions to leukemia-associated antigens in identical twins. Int. J. Cancer 1972, 9, 648–658.
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Cantoni, C.; Mingari, M.C.; Biassoni, R.; Moretta, L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 2001, 19, 197–223.
- Greenberg, A.H.; Hudson, L.; Shen, L.; Roitt, I.M. Antibody-dependent cell-mediated cytotoxicity due to a “null” lymphoid cell. Nat. New Biol. 1973, 242, 111–113.
- Herberman, R.B.; Nunn, M.E.; Holden, H.T.; Lavrin, D.H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer 1975, 16, 230–239.
- Borrego, F. The first molecular basis of the “missing self” hypothesis. J. Immunol. 2006, 177, 5759–5760.
- Ljunggren, H.G.; Karre, K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today 1990, 11, 237–244.
- Storkus, W.J.; Alexander, J.; Payne, J.A.; Dawson, J.R.; Cresswell, P. Reversal of natural killing susceptibility in target cells expressing transfected class I HLA genes. Proc. Natl. Acad. Sci. USA 1989, 86, 2361–2364.
- Storkus, W.J.; Alexander, J.; Payne, J.A.; Cresswell, P.; Dawson, J.R. The alpha 1/alpha 2 domains of class I HLA molecules confer resistance to natural killing. J. Immunol. 1989, 143, 3853–3857.
- Karlhofer, F.M.; Ribaudo, R.K.; Yokoyama, W.M. MHC Class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature 358: 66-70, 1992. J. Immunol. 2006, 177, 5761–5765.
- Yokoyama, W.M. The search for the missing ‘missing-self’ receptor on natural killer cells. Scand. J. Immunol. 2002, 55, 233–237.
- Moretta, A.; Vitale, M.; Bottino, C.; Orengo, A.M.; Morelli, L.; Augugliaro, R.; Barbaresi, M.; Ciccone, E.; Moretta, L. P58 molecules as putative receptors for major histocompatibility complex (MHC) class I molecules in human natural killer (NK) cells. Anti-p58 antibodies reconstitute lysis of MHC class I-protected cells in NK clones displaying different specificities. J. Exp. Med. 1993, 178, 597–604.
- Boudreau, J.E.; Hsu, K.C. Natural Killer Cell Education and the Response to Infection and Cancer Therapy: Stay Tuned. Trends Immunol. 2018, 39, 222–239.
- Fauriat, C.; Long, E.O.; Ljunggren, H.G.; Bryceson, Y.T. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010, 115, 2167–2176.
- Takeda, K.; Hayakawa, Y.; Smyth, M.J.; Kayagaki, N.; Yamaguchi, N.; Kakuta, S.; Iwakura, Y.; Yagita, H.; Okumura, K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat. Med. 2001, 7, 94–100.
- Backes, C.S.; Friedmann, K.S.; Mang, S.; Knörck, A.; Hoth, M.; Kummerow, C. Natural killer cells induce distinct modes of cancer cell death: Discrimination, quantification, and modulation of apoptosis, necrosis, and mixed forms. J. Biol. Chem. 2018, 293, 16348–16363.
- Bryceson, Y.T.; March, M.E.; Barber, D.F.; Ljunggren, H.G.; Long, E.O. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J. Exp. Med. 2005, 202, 1001–1012.
- Bryceson, Y.T.; Ljunggren, H.G.; Long, E.O. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood 2009, 114, 2657–2666.
- Vyas, Y.M.; Mehta, K.M.; Morgan, M.; Maniar, H.; Butros, L.; Jung, S.; Burkhardt, J.K.; Dupont, B. Spatial organization of signal transduction molecules in the NK cell immune synapses during MHC class I-regulated noncytolytic and cytolytic interactions. J. Immunol. 2001, 167, 4358–4367.
- Orange, J.S.; Harris, K.E.; Andzelm, M.M.; Valter, M.M.; Geha, R.S.; Strominger, J.L. The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc. Natl. Acad. Sci. USA 2003, 100, 14151–14156.
- Kim, H.S.; Das, A.; Gross, C.C.; Bryceson, Y.T.; Long, E.O. Synergistic signals for natural cytotoxicity are required to overcome inhibition by c-Cbl ubiquitin ligase. Immunity 2010, 32, 175–186.
- Das, A.; Long, E.O. Lytic granule polarization, rather than degranulation, is the preferred target of inhibitory receptors in NK cells. J. Immunol. 2010, 185, 4698–4704.
- Treanor, B.; Lanigan, P.M.; Kumar, S.; Dunsby, C.; Munro, I.; Auksorius, E.; Culley, F.J.; Purbhoo, M.A.; Phillips, D.; Neil, M.A.; et al. Microclusters of inhibitory killer immunoglobulin-like receptor signaling at natural killer cell immunological synapses. J. Cell Biol. 2006, 174, 153–161.
- Bhat, R.; Watzl, C. Serial killing of tumor cells by human natural killer cells--enhancement by therapeutic antibodies. PLoS ONE 2007, 2, 326.
- Long, E.O. Tumor cell recognition by natural killer cells. Semin. Cancer Biol. 2002, 12, 57–61.
- Orr, M.T.; Lanier, L.L. Natural killer cell education and tolerance. Cell 2010, 142, 847–856.
- Anfossi, N.; André, P.; Guia, S.; Falk, C.S.; Roetynck, S.; Stewart, C.A.; Breso, V.; Frassati, C.; Reviron, D.; Middleton, D.; et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 2006, 25, 331–342.
- Vey, N.; Karlin, L.; Sadot-Lebouvier, S.; Broussais, F.; Berton-Rigaud, D.; Rey, J.; Charbonnier, A.; Marie, D.; André, P.; Paturel, C.; et al. A phase 1 study of lirilumab (antibody against killer immunoglobulin-like receptor antibody KIR2D.; IPH2102) in patients with solid tumors and hematologic malignancies. Oncotarget 2018, 9, 17675–17688.
- Vey, N.; Bourhis, J.-H.; Boissel, N.; Bordessoule, D.; Prebet, T.; Charbonnier, A.; Etienne, A.; Andre, P.; Romagné, F.; Benson, N.; et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood 2012, 120, 4317–4323.
- Tinker, A.V.; Hirte, H.W.; Provencher, D.M.; Butler, M.O.; Ritter, H.; Tu, D.; Azim, H.A.; Paralejas, P.; Grenier, N.; Hahn, S.-A.; et al. Dose-ranging and cohort-expansion study of monalizumab (IPH2201) in patients with advanced gynecologic malignancies: A trial of the canadian cancer trials group (CCTG): IND221. Clin. Cancer Res. 2019, 25, 6052–6060.
- Chan, V.W.; Lowell, C.A.; DeFranco, A.L. Defective negative regulation of antigen receptor signaling in Lyn-deficient B lymphocytes. Curr. Biol. 1998, 8, 545–553.
- Burshtyn, D.; Scharenberg, A.M.; Wagtmann, N.; Rajagopalan, S.; Berrada, K.; Yi, T.; Kinet, J.-P.; Long, E.O. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitor receptor. Immunity 1996, 4, 77–85.
- Binstadt, B.; Brumbaugh, K.M.; Dick, C.J.; Scharenberg, A.M.; Williams, B.L.; Colonna, M.; Lanier, L.L.; Kinet, J.-P.; Abraham, R.T.; Leibson, P.J. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity 1996, 5, 629–638.
- Jakubowiak, A.J.; Benson, D.M.; Bensinger, W.; Siegel, D.S.; Zimmerman, T.M.; Mohrbacher, A.; Richardson, P.G.; Afar, D.E.; Singhal, A.K.; Anderson, K.C. Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. J. Clin. Oncol. 2012, 30, 1960–1965.
- Lonial, S.; Vij, R.; Harousseau, J.-L.; Facon, T.; Moreau, P.; Mazumder, A.; Kaufman, J.L.; Leleu, X.; Tsao, L.C.; Westland, C.; et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J. Clin. Oncol. 2012, 30, 1953–1959.
- Collins, S.M.; Bakan, C.E.; Swartzel, G.D.; Hofmeister, C.C.; Efebera, Y.A.; Kwon, H.; Starling, G.C.; Ciarlariello, D.; Bhaskar, S.; Briercheck, E.L.; et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: Evidence for augmented NK cell function complementing ADCC. Cancer Immunol. Immunother. 2013, 62, 1841–1849.
- Ernst, D.; Williams, B.A.; Wang, X.-H.; Yoon, N.; Kim, K.-P.; Chiu, J.; Luo, Z.J.; Hermans, K.G.; Krueger, J.; Keating, A. Humanized anti-CD123 antibody facilitates NK cell antibody-dependent cell-mediated cytotoxicity (ADCC) of Hodgkin lymphoma targets via ARF6/PLD-1. Blood Cancer, J. 2019, 9, 6.
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 2020, 382, 545–553.
- Xiao, L.; Cen, D.; Gan, H.; Sun, Y.; Huang, N.; Xiong, H.; Jin, Q.; Su, L.; Liu, X.; Wang, K.; et al. Adoptive transfer of NKG2D CAR mRNA-engineered natural killer cells in colorectal cancer patients. Mol. Ther. 2019, 27, 1114–1125.
- Deng, X.; Gao, F.; Li, N.; Li, Q.; Zhou, Y.; Yang, T.; Cai, Z.; Du, P.; Chen, F.; Cai, J. Antitumor activity of NKG2D CAR-T cells against human colorectal cancer cells in vitro and in vivo. Am. J. Cancer Res. 2019, 9, 945–958.
- Fernández, L.; Fernández, A.; Mirones, I.; Escudero, A.; Cardoso, L.; Vela, M.; Lanzarot, D.; De Paz, R.; Leivas, A.; Gallardo, M.; et al. GMP-compliant manufacturing of NKG2D CAR memory T cells using CliniMACS prodigy. Front. Immunol. 2019, 10, 2361.
This entry is offline, you can click here to edit this entry!
