Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Happiness is a feeling, an immediate experience, not a cognitive construct. It is based on activity in the brain’s neurobiological reward and motivation systems, which have been retained in evolution.
- happiness
- brain
- U-curve
- motivational salience
- reward
- life satisfaction
- subjective well-being
- aging
- mindfulness
- contemplative practice
1. Introduction
Research on happiness, especially with regard to its psychosocial as well as biological implications or its differentiation from satisfaction have gained momentum in recent years [1][2][3]. Today, happiness can be viewed as a highly relevant phenomenon: Happiness “makes sense” from a biological point of view and is not restricted to humans in principle [4]. Hence, happiness is not “intellectual”; it is not constructed or cognitive. Primarily, it is a feeling, not a thought, and it is also physical at the same time, that is, based on the motivational and reward system inside the brain [5][6]. Here, cognitive or evaluative parts are downstream, i.e., secondary or associative in nature [7].
The biology of happiness follows rather objective criteria. There is an overarching goal (e.g., survival, reproduction, behavior control [7][8]) that, if necessary, can override subjective or individual goals. Ideally, however, the primary (objective) and the secondary (subjective, subsequent) goals are aligned, that is, well-coordinated with one another. With regard to these secondary parts, study participants give different statements in surveys on the subject of happiness: When asked about their secondary assessment of happiness, respondents give answers that frequently diverge from those who had been queried about their primary feelings, i.e., about the direct experience in actual (happy) moments (c.f., subjective well-being (SWB) as a cognitive construct in contrast to the current emotional state or the mood in any given, immediate moment) [7][9][10].
Hence, happiness is not just a “good feeling”. Much more, it carries along strong biological cues and values. It provides inner guidelines, i.e., directions, and lets humans plan or implement behaviors from which researchers hope to derive a desired (beneficial) outcome [2][5][6][8]. Therefore, the feeling of happiness—if based on an experience that has already been made before (i.e., memorized, conditioned, and also linked to it: a positive expectation for the future)—is an efficient way of integrating or automating experiences made earlier into behavioral or treatment concepts that can be quickly implemented and activated (i.e., short-cut) [8][11][12].
Happiness as a biological concept was passed on genetically and further developed in evolution [4][7][13][14][15]. It has a biomolecular basis yet is used for autoregulation and survival (of the individual and of the species) [5][7][12][16][17]. Happiness can thus be measured (e.g., in the brain, blood; see below) and is associated with biophysiological changes in the body [7][18][19][20][21][22][23][24]. At its core, happiness, as is the case with the entire body (the living organism), is dynamic by nature and is subject to cyclical or internal “maturation processes” (see below). Thus, an interesting question coupled to this is whether happiness or its trajectories can actually be trained, i.e., actively be altered, for example, through positive psychology programs, contemplative or meditative practices.
Happiness as a natural, biologically “meaningful” phenomenon—a positive, pleasurable, or rewarding state that makes individuals feel, i.e., emotionally realize, an inner “calling”: a confirmation to show what is supposedly good or biologically beneficial for us—in this moment or over time. Happiness thus (invisibly) controls people's behavior. People's SWB is based on this, yet subsequently dependent on it.
Happiness is therefore neurobiologically “produced” in the brain by the reward and motivation systems. Researchers surmise that each of these “happy” mood states has a specific representation—i.e., a corresponding, analogous activation pattern—in the brain (see below). Since these states (patterns and pathways) are also related, e.g., to addiction or other external phenomena (i.e., drugs) [5][7], there can be negative, less beneficial interactions with behavior regarding the fact that these do not necessarily always have to be medically healthy (see below). This applies in particular to sub-forms of happiness, such as those associated with ecstasy or wanting (type A), or those directly related to stress (type B)—as it will be described in the next section.
Hence, the “hardware” for happiness is innate (albeit variable, in moderation), yet the “software” or operating systems are regularly updated through their use: what makes people happy is individual (to a large extent), but the structures and functions seem universal.
2. Three Types of Motivation and Rewarding (“Happy”) States
As stated above, happiness describes a feeling that is manifested biophysiologically in the brain, mind, and body. The feeling that comes along with it serves to memorize its initial occurrence and its antecedent, i.e., the contexts that led to or accompanied it. By this mechanism, “happy events” not only become conditioned and reinforced (i.e., learning and wanting to experience them again) yet they also become stored in memory together with an emotional “tag” for better and faster retrieval of the stored information later on, i.e., when it is biologically required or circumstances trigger memories of the initial event [7].
As with happiness, behaviors (and so: lifestyles, health behaviors) are shaped by implicit emotions and autonomous, unconscious processes (i.e., non-cognitive motives) rather than by metacognition or cognitive willpower (e.g., see [8][25][26][27][28][29]). In fact, health behaviors that are experienced as pleasant are more likely to be repeated (e.g., [5][30][31][32][33]). To better understand the underlying mechanisms of reward and behavioral motivation, including their neurobiological significance, I suggest distinguishing between three types of motivational states, namely, (A) approach motivation, (B) avoidance motivation, and (C) assertion motivation (Figure 1). The rationale behind this distinction and their implications will be explained in the following.
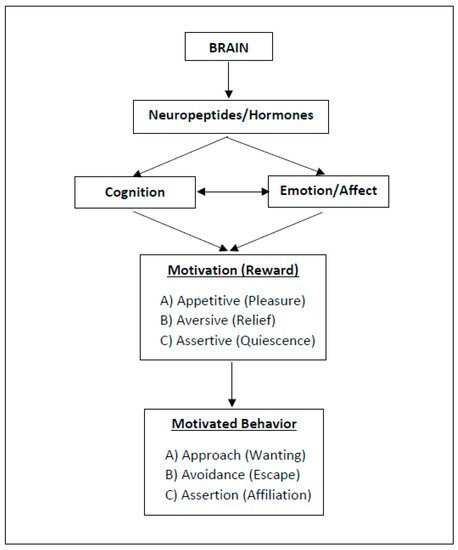
Figure 1. Three Types of Motivation and Reward (ABC Model). Motivational salience is the attribute of a stimulus and can be appetitive, aversive, or assertive. The related reward serves to steer biologically relevant core behaviors (for neurobiological implications, references: see text).
Motivation describes processes that represent the core of biological, cognitive, and social regulation [5], including the regulation of intensity of behavior that leads to the attainment of a particular goal or stimulus [34]. Researchers define stimuli as concrete physical objects, mental representations or memories of such objects, abstract concepts, or possibilities that are expected to occur in the future [35][36][37]. The pursuit (effect) of stimuli is based on affects (or “basic emotions”, see [38][39]) and can be automatic and occur with or without awareness. When cognitively processed, researchers may call these stimuli “goals”. Thus, behavior can be stimulus-driven (affective) or goal-directed (cognitive) [40], with the prior occurring most often (e.g., [8]).
Motivational salience is the attribute of a stimulus and can be appetitive, aversive [34], or assertive [7][41]. However, the existing literature does not sufficiently differentiate between assertive and appetitive motivation; usually, both fall under the umbrella term “approach motivation”. As noted earlier, I now suggest distinguishing three types of motivational processes (ABC), since they involve different physiological/neurobiological mechanisms (Figure 1).
2.1. Approach Motivation
Approach motivation, or appetitive (incentive) salience, is directed towards stimuli or goals that are related to positive, hedonic, pleasant processes [5][36][42] and functionally linked to the wanting system, i.e., reward expectation, performance and action [7]. This type of motivation is sometimes also referred to as “wanting motivation” [7].
If approach motivation (appetite, wanting) has led to the achievement of a stimulus or goal, a reward is experienced as a pleasant feeling (which, depending on the intensity of the experience, can also go unnoticed). The stimulus (goal) itself does not serve as a reward. Instead, reinforcement occurs either via psychological and related neurobiological processes that take place during one’s anticipatory (expectant) state and/or as a response to the actual stimulus or goal [43][44].
Individuals continually evaluate stimuli and determine them to be either beneficial or detrimental [36]. These evaluations are often perceived as basic affective experiences [36][45][46][47][48]. Simply put, approach motivation emphasizes the expectation of a reward in form of pleasant feelings, or positive affect, e.g., joy, pleasure, and excitement.
Underlying the concepts of motivation are physiological mechanisms that occur in brain areas distinct from other sensory or cognitive areas [5][7][49]. An integral part of the central nervous system (CNS), approach motivation and reward systems are neurons that have their principal origin in the ventral tegmental area (VTA), located in the midbrain [5][12]. These neurons send projections, e.g., to the frontolimbic brain, mostly to the nucleus accumbens (NAcc) [50][51]. By this, the midbrain and the frontolimbic systems are “wired” together; this is known as mesolimbic coupling. When a stimulus is paired with a reward initially or when a reward is anticipated in response to a stimulus based on past experience, via the mesolimbic pathway with its essential neurotransmitter dopamine, VTA and NAcc become connected and activated [52][53], i.e., “dopaminergic activation promotes positive feelings” [7]. The NAcc hence signals the desire to obtain a reward and the degree of effort to do so, i.e., it determines appetitive motivational salience. In addition, reward is also measured and regulated by the VTA-NAcc pathway—it signals to other brain areas how rewarding an activity is [5]. The intensity of an expected reward, consequently, determines how likely it is that a person will remember and repeat it [12]. Here, the hippocampus, as part of the (para)limbic system, serves as the point of entry for experiences to be recognized and remembered [5][54].
Additionally, by also involving the amygdala, people's brain categorizes and remembers experiences as pleasant or detrimental within the endogenous reward system, which in turn facilitates the pairing between experiences and other stimuli [12][50][51]. Such information is also processed via the mesocortical (mesofrontal) dopamine pathway in the frontal cortex, which is integral to weighing one’s personal “costs” (disadvantages) against the likelihood of reward. Whether the behavior is ultimately performed or not is determined by these dynamic processes of deliberation, with the hedonic value driving the wanting, i.e., the appetite and approach motivation [8][12].
2.2. Avoidance Motivation
Aversive motivational, or negatively-valenced “fearful” salience, is related to the avoidance of pain or threat (i.e., threat avoidance) and punishment (i.e., consequences that diminish the chance of the reoccurrence of a behavior), and it corresponds with the fight-flight-freeze system (i.e., stress physiology, stress response) [5][7][42][55]. It is usually triggered by an aversive stimulus and is motivated by a desire to attain relief from undesirable circumstances.
Punishment, which is also linked to a reduced response strength (i.e., passive avoidance), can further be distinguished from negative reinforcement, the latter of which is related to an increased response strength (i.e., active avoidance) [44]. Thus, in comparison to active fear reactions (e.g., fight or flight) resulting from fearful stimuli, passive responses (e.g., freezing) can also occur [56].
Anxiety, fear, and disgust are examples of negative affects associated with avoidance motivation [7][36][45][57][58][59]. Functionally embedded in the stress system, avoidance motivation is related to increased sympathetic and stress activity (i.e., activated stress responses), including the release of cortisol, (nor-) adrenaline, as well as, e.g., opioids and vasopressin [7]. Anatomically, it is rooted in the lower limbic system, primarily in the amygdala and hypothalamus (connected to the pituitary gland) [12].
Two central signaling pathways are activated when threat is anticipated: One pathway involves the hypothalamus, which receives its impulses from cortical, subcortical, limbic and brainstem areas, and leads to the pituitary gland, where (pre-) hormones are released into the blood, triggering the release, e.g., of cortisol from the adrenal cortex. Cortisol is particularly responsible for providing energy for “fight and flight” [7]. The other pathway acts via the sympathetic autonomous nervous system. Here, too, the hypothalamus, but above all the brainstem and the brain’s catecholamine systems, are crucial. From here, nerve impulses are directly (neuronally) transmitted to the peripheral organs and the adrenal medulla. The stress hormone adrenaline (or noradrenaline) is released from there. This second pathway is the faster, more direct one, and particularly affects the circulatory and organ functions [7][12]. In addition, the freeze response, as mentioned above, is functionally embedded in the CNS amygdala circuitry [60].
In the context of successful passive or active threat avoidance, the modulation of the various signaling pathways involved ultimately leads to the perception of relief, which is a positive, low-arousal basic emotion/affect [61][62], corresponding with one’s original (conscious or unconscious) intention, i.e., to survive. Indeed, when the aversive stimulus is discontinued, actively or passively, relief occurs [63]. Psychologically, relief results from the reduced impact of a negative stimulus and can be experienced as relaxation and/or reward, i.e., “no more stress” [6][12][64][65].
Relief is also experienced when the activity of the amygdala is reduced [7][12]. This, too, signals (or is the biological consequence of) less or “no stress”—which, again, could be an active or a passive process. Furthermore, researchers see a connection between approach and avoidance motivation systems here: Results, e.g., from functional magnetic resonance imaging (fMRI)-based studies suggest that corresponding brain areas are activated during relief and other positive affects (e.g., see [66][67]).
2.3. Assertion Motivation
As described above, the majority of previously published research on motivation and reward does not distinguish between behavior driven by approach versus assertion motivation. In fact, these constructs or states are often confounded or merged with each other [41], even though they reflect different neurobiological processes, are originating from distinct areas of the brain, and have different behavioral consequences [7][8]. Assertion motivation, or assertive salience, is associated with the “non-wanting” system or “non-wanting motivation”, hence with inaction (i.e., staying), acceptance or contentedness, and quiescence. It characterizes the motivation to maintain a certain condition or state [7][41]; the associated positive valence is contentment. The assertion motivation differs from the approach motivation with regard to the affective qualities involved [7][41]. Furthermore, assertion motivation can also be distinguished from approach motivation in terms of different automatic responses and behavioral outcomes.
Assertion motivation is based on the lack of a goal-directed, recognizable action (neither having to get somewhere nor having to get away), because one has purposefully (explicitly) or unconsciously (implicitly) consented to remaining in the current state, for example, a newly habituated health behavior. Consenting to the current state can be experienced as being “perfectly happy”, or content, having no intention to change it or move away from it, or shift one’s attention towards some other thing or place. Hence, by being in congruence with or in full acceptance of the present moment, this state of non-wanting also entails a feeling of “mindful” connectedness (an alignment with the “here and now”), and the underlying system is thus sometimes called the “affiliation system” [7]. Unsurprisingly, mindfulness or meditation practices seem to facilitate this kind of experience [68][69].
Functionally embedded within the parasympathetic autonomous nervous system, the assertive motivational state is linked to increased parasympathetic or vagus nerve activity and is therefore associated with a physio-psychological down-regulation, and states of relaxation [70][71][72][73]. At the level of neurotransmitter systems, assertive salience is associated with endogenous opiates, oxytocin, acetylcholine, serotonin, as well as endocannabinoid signaling [7][12][20][69][74][75][76]. Unlike approach and avoidance motivation systems, the assertion motivation system is not characterized by an involvement of dopamine—instead, it is linked to the absence or inhibition of dopaminergic activation. Thus, individuals here experience no motivation to change the status quo by generating or avoiding new experiences through behavior change. Brain areas involved in the activation of assertive motivation include, but are not limited to, the midbrain, the vagus areas, brainstem, cingulum, hippocampus and ventral striatum, as well as the hypothalamus and the pituitary gland [7].
This entry is adapted from the peer-reviewed paper 10.3390/biology11060843
References
- Jackson, J.L. The Pursuit and Maintenance of Happiness. N. Engl. J. Med. 2021, 385, 1327–1328.
- Steptoe, A. Happiness and Health. Annu. Rev. Public Health 2019, 40, 339–359.
- Helliwell, J.F.; Aknin, L.B. Expanding the social science of happiness. Nat. Hum. Behav. 2018, 2, 248–252.
- Webb, L.E.; Veenhoven, R.; Harfeld, J.L.; Jensen, M.B. What is animal happiness? Ann. N. Y. Acad. Sci. 2019, 1438, 62–76.
- Esch, T.; Stefano, G.B. The neurobiology of pleasure, reward processes, addiction and their health implications. Neuroendocrinol. Lett. 2004, 25, 235–251.
- Esch, T.; Stefano, G.B. Endogenous reward mechanisms and their importance in stress reduction, exercise and the brain. Arch. Med. Sci. 2010, 6, 447–455.
- Esch, T. The Neurobiology of Happiness, 3rd ed.; Thieme: Stuttgart, Germany, 2017.
- Michaelsen, M.M.; Esch, T. Motivation and reward mechanisms in health behavior change processes. Brain Res. 2021, 1757, 147309.
- Karwetzky, C.; Werdecker, L.; Esch, T. What Matters Most in Life? A German Cohort Study on the Sources of Meaning and Their Neurobiological Foundations in Four Age Groups. Front. Psychol. 2021, 12, 777751.
- Karwetzky, C.; Michaelsen, M.M.; Werdecker, L.; Esch, T. The U-curve of happiness revisited: Correlations and differences in life satisfaction over the span of life—an empirical evaluation based on data from 1,597 individuals aged 12 to 94 in Germany. Front. Psychol. 2022, 13, 837638.
- Michaelsen, M.M.; Esch, T. Functional mechanisms of health behavior change techniques: A conceptual review. Front. Psychol. 2022, 13, 725644.
- Esch, T.; Stefano, G.B. The neurobiology of stress management. Neuroendocrinol. Lett. 2010, 31, 19–39.
- Buss, D.M. The evolution of happiness. Am. Psychol. 2000, 55, 15–23.
- Loonen, A.J.M.; Ivanova, S.A. Circuits regulating pleasure and happiness: Evolution and role in mental disorders. Acta Neuropsychiatr. 2018, 30, 29–42.
- Loonen, A.J.; Ivanova, S.A. Circuits Regulating Pleasure and Happiness: The Evolution of the Amygdalar-Hippocampal-Habenular Connectivity in Vertebrates. Front. Neurosci. 2016, 10, 539.
- Esch, T. Self-regulation as part of medicine. Dtsch Arztebl Int. 2016, 111, 2214–2220.
- Esch, T. Self-healing in health-care: Using the example of mind-body medicine. Bundesgesundheitsblatt Gesundh. Gesundh. 2020, 63, 577–585.
- Wang, Z.; Zhang, Y.; Li, Q.; Zou, Q.; Liu, Q. A road map for happiness: The psychological factors related cell types in various parts of human body from single cell RNA-seq data analysis. Comput. Biol. Med. 2022, 143, 105286.
- Feicht, T.; Wittmann, M.; Jose, G.; Mock, A.; von Hirschhausen, E.; Esch, T. Evaluation of a seven-week web-based happiness training to improve psychological well-being, reduce stress, and enhance mindfulness and flourishing: A randomized controlled occupational health study. Evid. Based Complement. Alternat. Med. 2013, 2013, 676953.
- Zak, P.J.; Curry, B.; Owen, T.; Barraza, J.A. Oxytocin release increases with age and is associated with life satisfaction and prosocial behaviors. Front. Behav. Neurosci. 2022, 16, 846234.
- Park, S.; Kahnt, T.; Dogan, A.; Strang, S.; Fehr, E.; Tobler, P.N. A neural link between generosity and happiness. Nat. Commun. 2017, 8, 15964.
- Rutledge, R.B.; Skandali, N.; Dayan, P.; Dolan, R.J. Dopaminergic modulation of decision making and subjective well-being. J. Neurosci. 2015, 35, 9811–9822.
- Burgdorf, J.; Panksepp, J. The neurobiology of positive emotions. Neurosci. Biobehav. Rev. 2006, 30, 173–187.
- Kikuchi, A.M.; Tanabe, A.; Iwahori, Y. A systematic review of the effect of L-tryptophan supplementation on mood and emotional functioning. J. Diet. Suppl. 2021, 18, 316–333.
- Hall, P.A.; Fong, G.T. Temporal self-regulation theory: A model for individual health behavior. Health Psychol. Rev. 2007, 1, 6–52.
- Kahneman, D.; Tversky, A. Prospect Theory: An Analysis of Decision under Risk. Econometrica 1979, 47, 263–291.
- Marteau, T.M.; Hollands, G.J.; Fletcher, P.C. Changing Human Behavior to Prevent Disease: The Importance of Targeting Automatic Processes. Science 2012, 337, 1492–1495.
- Sheeran, P.; Gollwitzer, P.M.; Bargh, J.A. Nonconscious processes and health. Health Psychol. 2013, 32, 460–473.
- Strack, F.; Deutsch, R. Reflective and impulsive determinants of social behavior. Pers. Soc. Psychol. Rev. 2004, 8, 220–247.
- Cohn, M.A.; Fredrickson, B.L. In search of durable positive psychology interventions: Predictors and consequences of long-term positive behavior change. J. Posit. Psychol. 2010, 5, 355–366.
- Fredrickson, B.L.; Arizmendi, C.; van Cappellen, P. Same-day, cross-day, and upward spiral relations between positive affect and positive health behaviours. Psychol. Health 2020, 36, 444–460.
- Rhodes, R.E.; Kates, A. Can the Affective Response to Exercise Predict Future Motives and Physical Activity Behavior? A Systematic Review of Published Evidence. Ann. Behav. Med. 2015, 49, 715–731.
- Woolley, K.; Fishbach, A. Immediate Rewards Predict Adherence to Long-Term Goals. Pers. Soc. Psychol. Bull. 2017, 43, 151–162.
- Puglisi-Allegra, S.; Ventura, R. Prefrontal/accumbal catecholamine system processes emotionally driven attribution of motivational salience. Rev. Neurosci. 2012, 23, 509–526.
- Eder, A.B.; Hommel, B. Anticipatory control of approach and avoidance: An ideomotor approach. Emot. Rev. 2013, 5, 276–280.
- Elliot, A.J.; Eder, A.B.; Harmon-Jones, E. Approach-avoidance motivation and emotion: Convergence and divergence. Emot. Rev. 2013, 5, 308–311.
- Scholer, A.A.; Higgins, E.T. Dodging monsters and dancing with dreams: Success and failure at different levels of approach and avoidance. Emot. Rev. 2013, 5, 254–258.
- Ekman, P.; Cordaro, D. What is meant by calling emotions basic. Emot. Rev. 2011, 3, 364–370.
- Ekman, P. Basic Emotions. In Handbook of Cognition and Emotion; Dalgleish, T., Power, M.J., Eds.; Wiley-Interscience: Hoboken, NJ, USA, 2005; pp. 45–60.
- Bourgeois, A.; Neveu, R.; Vuilleumier, P.; Chelazzi, L. How does awareness modulate goal-directed and stimulus-driven shifts of attention triggered by value learning? PLoS ONE 2016, 11, e0160469.
- McCall, C.; Singer, T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat. Neurosci. 2012, 15, 681–688.
- Bozarth, M. Pleasure systems in the brain. In Pleasure: The Politics and the Reality; Warburton, D.M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1994; pp. 5–14.
- Berridge, K.C.; Kringelbach, M.L. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology 2008, 199, 457–480.
- Schultz, W. Neuronal reward and decision signals: From theories to data. Physiol. Rev. 2015, 95, 853–951.
- Cacioppo, J.T.; Gardner, W.L.; Berntson, G.G. The affect system has parallel and integrative processing components: Form follows function. J. Pers. Soc. Psychol. 1999, 76, 839–855.
- Lang, P.J.; Bradley, M.M. Appetitive and defensive motivation: Goal-directed or goal-determined? Emot. Rev. 2013, 5, 230–234.
- Rolls, E.T. What are emotional states, and why do we have them? Emot. Rev. 2013, 5, 241–247.
- Schneirla, T.C. An evolutionary and developmental theory of biphasic processes underlying approach and withdrawal. In Nebraska Symposium on Motivation; Jones, M.R., Ed.; University Nebraska Press: Lincoln, NE, USA, 1959; pp. 1–42.
- Kringelbach, M.L. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat. Rev. Neurosci. 2005, 6, 691–702.
- Nestler, E.J. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2001, 2, 119–128.
- Nestler, E.J.; Malenka, R.C.; Hyman, S.E. Molecular Basis of Neuropharmacology; McGraw-Hill Medical: New York, NY, USA, 2001.
- Berridge, K.C. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology 2007, 191, 391–431.
- Smith, K.S.; Berridge, K.C.; Aldridge, J.W. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc. Natl. Acad. Sci. USA 2011, 108, E255–E264.
- Nestler, E.J.; Malenka, R.C. The addicted brain. Sci. Am. 2004, 290, 78–85.
- Seymour, B.; Singer, T.; Dolan, R. The neurobiology of punishment. Nat. Rev. Neurosci. 2007, 8, 300–311.
- Berridge, K.C. Evolving concepts of emotion and motivation. Front. Psychol. 2018, 9, 1647.
- Hirschberg, J.; Manning, C.D. Advances in natural language processing. Science 2015, 349, 261–266.
- Lang, P.J. The emotion probe: Studies of motivation and attention. Am. Psychol. 1995, 50, 372–385.
- Watson, D.; Wiese, D.; Vaidya, J.; Tellegen, A. The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. J. Pers. Soc. Psychol. 1999, 76, 820–838.
- LeDoux, J. Fear and the brain: Where have we been, and where are we going? Biol Psychiatry 1998, 44, 1229–1238.
- Krisam, M.; Meder, B.; von Philipsborn, P. Nudging in Primary Prevention: Overview and Perspectives for Germany. Gesundheitswesen 2017, 79, 117–123.
- Levenson, R.W. Basic emotion questions. Emot. Rev. 2011, 3, 379–386.
- Deutsch, R.; Smith, K.J.M.; Kordts-Freudinger, R.; Reichardt, R. How absent negativity relates to affect and motivation: An integrative relief model. Front. Psychol. 2015, 6, 152.
- Esch, T.; Stefano, G.B.; Fricchione, G.L. The therapeutic use of the relaxation response in stress-related diseases. Med. Sci. Monit. 2003, 9, RA23–RA34.
- Stefano, G.B.; Fricchione, G.L.; Esch, T. Relaxation: Molecular and physiological significance. Med. Sci. Monit. 2006, 12, HY21–HY31.
- Kim, J.; Lee, S.; Park, K.; Hong, I.; Song, B.; Son, G.; Park, H.; Kim, W.R.; Park, E.; Choe, H.K.; et al. Amygdala depotentiation and fear extinction. Proc. Natl. Acad. Sci. USA 2007, 104, 20955–20960.
- Sangha, S. Plasticity of fear and safety neurons of the amygdala in response to fear extinction. Front. Behav. Neurosci. 2015, 9, 354.
- Esch, T. Meditation in Complementary and Integrative Medicine: Taxonomy of Effects and Methods. Complement. Med. Res. 2021, 28, 185–188.
- Esch, T. The Neurobiology of Meditation and Mindfulness. In Meditation—Neuroscientific Approaches and Philosophical Implications/Springer Series: Studies in Neuroscience, Consciousness and Spirituality; Schmidt, S., Walach, H., Eds.; Springer International Publishing: New York, NY, USA, 2014; Volume 2, pp. 153–173.
- Esch, T.; Kream, R.M.; Stefano, G.B. Chromosomal processes in mind-body medicine: Chronic stress, cell aging, and telomere length. Med. Sci. Monit. Bas. Res. 2018, 24, 134–140.
- Stefano, G.B.; Benson, H.; Fricchione, G.L.; Esch, T. The Stress Response: Always Good and When It Is Bad; Medical Science International: New York, NY, USA, 2005.
- Stefano, G.B.; Esch, T. Integrative medical therapy: Examination of meditation’s therapeutic and global medicinal outcomes via nitric oxide. Int. J. Mol. Med. 2005, 16, 621–630.
- Esch, T.; Guarna, M.; Bianchi, E.; Zhu, W.; Stefano, G.B. Commonalities in the central nervous system’s involvement with complementary medical therapies: Limbic morphinergic processes. Med. Sci. Monit. 2004, 10, MS6–MS17.
- Esch, T. The neuronal basis of meditation and mindfulness. Sucht 2014, 60, 21–28.
- Stefano, G.B.; Esch, T.; Cadet, P.; Zhu, W.; Mantione, K.; Benson, H. Endocannabinoids as autoregulatory signaling molecules: Coupling to nitric oxide and a possible association with the relaxation response. Med. Sci. Monit. 2003, 9, RA63–RA75.
- Huffmeijer, R.; van Ijzendoorn, M.H.; Bakermans-Kranenburg, M.J. Ageing and oxytocin: A call for extending human oxytocin research to ageing populations—a mini review. Gerontology 2013, 59, 32–39.
This entry is offline, you can click here to edit this entry!
