The increased use of dental implants in oral rehabilitation has been followed by the development of new biomaterials as well as improvements in the performance of biomaterials already in use inspired by the properties of the tissues to be replaced. An implant is considered osseointegrated when there is no relative movement between the implant and the bone and no symptoms under a loading force. It is now known that surface topography is one of the key biomimetic factors that can directly affect the proliferation, structure, and alignment of human cells and their function and is also considered to be a critical determinant of cell adhesion.
- implant surface
- osseointegration
- dentistry
- oral surgery
- oral rehabilitation
- biomimetic
- dental implants
1. Introduction
- (1)
-
The initial tissue response;
- (2)
-
Peri-implant osteogenesis;
- (3)
-
Peri-implant bone remodelling.
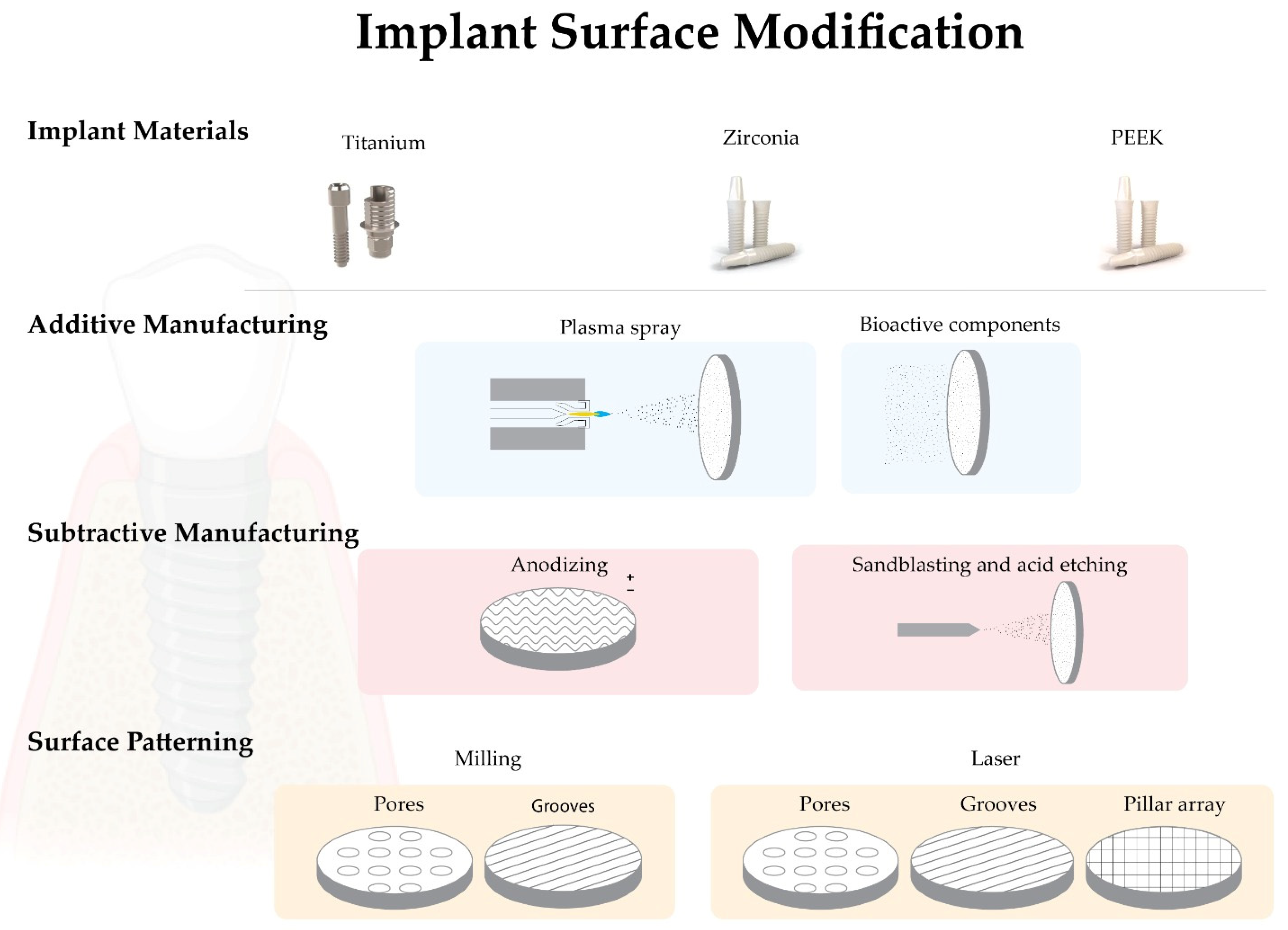
2. Dental Implants Base Materials
2.1. Titanium
2.2. Zirconia
3. Biomimetic Surface Properties
3.1. Topography
3.2. Roughness
4. Implant Surface Modifications
4.1. Biomimetic Surface Modifications—Additive Manufacturing
4.1.1. Plasma Spray
4.1.2. Addition of Bioactive Components
4.2. Biomimetic Surface Modifications—Subtractive Manufacturing
4.2.1. Anodizing
4.2.2. Blasting and/or Acid Etching
This entry is adapted from the peer-reviewed paper 10.3390/biomimetics7020074
References
- Londoño, J.J.; Ramos, A.M.; Correa, S.A.; Mesnard, M. Review of expandable dental implants. Br. J. Oral Maxillo-Facial Surg. 2021, 59, 546–554.
- Esposito, M.; Ardebili, Y.; Worthington, H.V. Interventions for replacing missing teeth: Different types of dental implants. Cochrane Database Syst. Rev. 2019, 2019, CD003815.
- Mombelli, A.; Müller, N.; Cionca, N. The epidemiology of peri-implantitis. Clin. Oral Implant. Res. 2012, 23, 67–76.
- Elias, C.N.; Oshida, Y.; Lima, J.H.C.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242.
- Gupta, R.; Gupta, N.; Weber, K.K.; Dental Implants. StatPearls 2021. Available online: https://europepmc.org/article/nbk/nbk470448 (accessed on 13 April 2022).
- Comisso, I.; Arias-Herrera, S.; Gupta, S. Zirconium dioxide implants as an alternative to titanium: A systematic review. J. Clin. Exp. Dent. 2021, 13, e511–e519.
- Bobbio, A. The first endosseous alloplastic implant in the history of man. Bull. Hist. Dent. 1972, 20, 1–6.
- Branemark, P. Vital microscopy of bone marrow in rabbit. Scand. J. Clin. Lab. Investig. 1959, 11, 1–82.
- Brånermark, P.; Adell, R.; Albrektsson, T.; Lekholm, U.; Lundkivist, S.; Rockler, B. Osseointegrated titanium fixtures in the treatment of edentulousness. Biomaterials 1983, 4, 25–28.
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746.
- Pellegrini, G.; Francetti, L.; Barbaro, B.; del Fabbro, M. Novel surfaces and osseointegration in implant dentistry. J. Inves-Tigative Clin. Dent. 2018, 9, e12349.
- McMahon, M.; Ye, S.; Pedrina, J.; Dlugolenski, D.; Stambas, J. Extracellular Matrix Enzymes and Immune Cell Biology. Front. Mol. Biosci. 2021, 8, 703868.
- Esposito, M.; Grusovin, M.G.; Worthington, H. Interventions for replacing missing teeth: Treatment of peri-implantitis. Cochrane Database Syst. Rev. 2012, 1, CD004970.
- Liñares, A.; Grize, L.; Muñoz, F.; Pippenger, B.E.; Dard, M.; Domken, O.; Blanco-Carrión, J. Histological assessment of hard and soft tissues sur-rounding a novel ceramic implant: A pilot study in the minipig. J. Clin. Periodontol. 2016, 43, 538–546.
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prostho-dontics. J. Prosthodont. Res. 2016, 60, 12–19.
- Brånemark, R.; Brånemark, P.I.; Rydevik, B.; Myers, R.R. Osseointegration in skeletal reconstruction and rehabilitation: A review. J. Rehabil. Res. Dev. 2001, 38, 175–181.
- Lee, J.W.Y.; Bance, M.L. Physiology of Osseointegration. Otolaryngol. Clin. N. Am. 2019, 52, 231–242.
- Ajami, E.; Fu, C.; Wen, H.B.; Bassett, J.; Park, S.J.; Pollard, M. Early Bone Healing on Hydroxyapatite-Coated and Chemically-Modified Hydrophilic Implant Surfaces in an Ovine Model. Int. J. Mol. Sci. 2021, 22, 9361.
- le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854.
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969.
- Amengual-Peñafiel, L.; Córdova, L.A.; Jara-Sepúlveda, M.C.; Brañes-Aroca, M.; Marchesani-Carrasco, F.; Cartes-Velásquez, R. Osteoimmunology drives dental implant osseointegration: A new paradigm for implant dentistry. Jpn. Dent. Sci. Rev. 2021, 57, 12–19.
- Chawla, A. Control of Macrophage Activation and Function by PPARs. Circ. Res. 2010, 106, 1559–1569.
- Kim, J.; Adachi, T. Cell-fate decision of mesenchymal stem cells toward osteocyte differentiation is committed by spheroid culture. Sci. Rep. 2021, 11, 13204.
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57.
- Irandoust, S.; Müftü, S. The interplay between bone healing and remodeling around dental implants. Sci. Rep. 2020, 10, 4335.
- Zarb, G.A.; Schmitt, A. The longitudinal clinical effectiveness of osseointegrated dental implants in anterior partially edentulous patients. Implant Dent. 1993, 6, 189–196.
- Sykaras, N.; Iacopino, A.M.; Marker, V.A.; Triplett, R.G.; Woody, R.D. Implant materials, designs, and surface topographies: Their effect on osseointegration. A literature review. Int. J. Oral Maxillofac. Implant. 2000, 15, 675–690.
- Steinemann, S. Titanium–The material of choice? Periodontol 2000 1998, 17, 7–21.
- Huang, Y.S.; McGowan, T.; Lee, R.; Ivanovski, S. 7.23 Dental Implants: Biomaterial Properties Influencing Osseointegration. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; Volume 7, pp. 444–466. ISBN 9780081006924.
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000 2017, 73, 22–40.
- Sharma, A.; Waddell, J.N.; Li, K.C.; Sharma, L.A.; Prior, D.J.; Duncan, W.J. Is titanium–zirconium alloy a better alternative to pure titanium for oral implant? Composition, mechanical properties, and microstructure analysis. Saudi Dent. J. 2021, 33, 546–553.
- Contaldo, M.; De Rosa, A.; Nucci, L.; Ballini, A.; Malacrinò, D.; La Noce, M.; Inchingolo, F.; Xhajanka, E.; Ferati, K.; Bexheti-Ferati, A.; et al. Titanium Functionalized with Polylysine Homopolymers: In Vitro Enhancement of Cells Growth. Materials 2021, 14, 3735.
- Yin, L.; Nakanishi, Y.; Alao, A.R.; Song, X.F.; Abduo, J.; Zhang, Y. A Review of Engineered Zirconia Surfaces in Biomedical Applications. In Procedia CIRP; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 65, pp. 284–290.
- Webber, L.P.; Chan, H.-L.; Wang, H.-L. Will Zirconia Implants Replace Titanium Implants? Appl. Sci. 2021, 11, 6776.
- Sivaraman, K.; Chopra, A.; Narayan, A.I.; Balakrishnan, D. Is zirconia a viable alternative to titanium for oral implant? A critical review. J. Prosthodont. Res. 2018, 62, 121–133.
- Chopra, D.; Jayasree, A.; Guo, T.; Gulati, K.; Ivanovski, S. Advancing dental implants: Bioactive and therapeutic modifications of zirconia. Bioact. Mater. 2021, 13, 161–178.
- Hafezeqoran, A.; Koodaryan, R. Effect of Zirconia Dental Implant Surfaces on Bone Integration: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2017, 2017, 9246721.
- Assal, P.A. The osseointegration of zirconia dental implants. Schweiz Monatsschr. Zahnmed. 2013, 123, 644–654.
- Cooper, L.F. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J. Prosthet. Dent. 2000, 84, 522–534.
- Rompen, E.; Domken, O.; Degidi, M.; Pontes, A.E.F.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implant. Res. 2006, 17 (Suppl. S2), 55–67.
- Accioni, F.; Vázquez, J.; Merinero, M.; Begines, B.; Alcudia, A. Latest Trends in Surface Modification for Dental Implantology: Innovative Developments and Analytical Applications. Pharmaceutics 2022, 14, 455.
- Albrektsson, T.; Wennerberg, A. The Impact of Oral Implants-Past and Future, 1966–2042. J. Can. Dent. Assoc. 2005, 71, 327.
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902.
- Zhu, X.; Chen, J.; Scheideler, L.; Altebaeumer, T.; Geis-Gerstorfer, J.; Kern, D. Cellular reactions of osteoblasts to micron- and sub-micron-scale porous structures of titanium surfaces. Cells Tissues Organs 2004, 178, 13–22.
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184.
- Ketabi, M.; DePorter, D. The effects of laser microgrooves on hard and soft tissue attachment to implant collar surfaces: A literature review and interpretation. Int. J. Periodontics Restor. Dent. 2013, 33, e145–e152.
- Taniguchi, Y.; Kakura, K.; Yamamoto, K.; Kido, H.; Yamazaki, J. Accelerated Osteogenic Differentiation and Bone Formation on Zirconia with Surface Grooves Created with Fiber Laser Irradiation. Clin. Implant Dent. Relat. Res. 2015, 18, 883–894.
- Cervino, G.; Meto, A.; Fiorillo, L.; Odorici, A.; Meto, A.; D’Amico, C.; Oteri, G.; Cicciù, M. Surface Treatment of the Dental Implant with Hyaluronic Acid: An Overview of Recent Data. Int. J. Environ. Res. Public Health 2021, 18, 4670.
- von Wilmowsky, C.; Moest, T.; Nkenke, E.; Stelzle, F.; Schlegel, K.A. Implants in bone: Part I. A current overview about tissue response, surface modifications and future perspectives. Oral Maxillofac. Surg. 2014, 18, 243–257.
- Ventre, M.; Natale, C.F.; Rianna, C.; Netti, P.A. Topographic cell instructive patterns to control cell adhesion, polarization and migration. J. R. Soc. Interface 2014, 11, 20140687.
- Hotchkiss, K.M.; Sowers, K.T.; Olivares-Navarrete, R. Novel in vitro comparative model of osteogenic and inflammatory cell response to dental implants. Dent. Mater. 2019, 35, 176–184.
- Ponche, A.; Bigerelle, M.; Anselme, K. Relative influence of surface topography and surface chemistry on cell response to bone implant materials. Part 1: Physico-chemical effects. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1471–1486.
- Santos, P.M.D.; Julio, E.N.B.S. A state-of-the-art review on roughness quantification methods for concrete surfaces. Constr. Build. Mater. 2013, 38, 912–923.
- Chen, S.; Feng, R.; Zhang, C.; Zhang, Y. Surface roughness measurement method based on multi-parameter modeling learning. Measurement 2018, 129, 664–676.
- Becker, S.T.; Beck-Broichsitter, B.E.; Rossmann, C.M.; Behrens, E.; Jochens, A.; Wiltfang, J. Long-term Survival of Straumann Dental Implants with TPS Surfaces: A Retrospective Study with a Follow-up of 12 to 23 Years. Clin. Implant Dent. Relat. Res. 2015, 18, 480–488.
- Åstrand, P.; Anzén, B.; Karlsson, U.; Sahlholm, S.; Svardstrom, P.; Hellem, S. Nonsubmerged Implants in the Treatment of the Edentulous Upper Jaw: A Prospective Clinical and Radiographic Study of ITI Implants—Results after 1 Year. Clin. Implant. Dent Relat. Res. 2000, 2, 166–174.
- Anselme, K.; Bigerelle, M.; Noel, B.; Dufresne, E.; Judas, D.; Iost, A.; Hardouin, P. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 2000, 49, 155–166.
- Blank, E.; Grischke, J.; Winkel, A.; Eberhard, J.; Kommerein, N.; Doll, K.; Yang, I.; Stiesch, M. Evaluation of biofilm colonization on multi-part dental implants in a rat model. BMC Oral Health 2021, 21, 313.
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918.
- Liao, H.; Fatash, B.; Li, J. Stability of hydroxyapatite-coatings on titanium oral implants (IMZ) 2 retrieved cases. Clin. Oral Implant. Res. 1997, 8, 68–72.
- Gittens, R.A.; Olivares-Navarrete, R.; Cheng, A.; Anderson, D.M.; McLachlan, T.; Stephan, I.; Geis-Gerstofer, J.; Sandhage, K.H.; Fedorov, A.G.; Rupp, F.; et al. The roles of titanium surface mi-cro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 2013, 9, 6268–6277.
- Lellouche, J.; Friedman, A.; Gedanken, A.; Banin, E. Antibacterial and antibiofilm properties of yttrium fluoride nanoparticles. Int. J. Nanomed. 2012, 7, 5611–5624.
- Wyszogrodzka, G.; Marszałek, B.; Gil, B.; Dorozyński, P. Metal-organic frameworks: Mechanisms of antibacterial action and po-tential applications. Drug Discov. Today 2016, 21, 1009–1018.
- Mondal, D.; Nguyen, L.; Oh, I.-H.; Lee, B.-T. Microstructure and biocompatibility of composite biomaterials fabricated from titanium and tricalcium phosphate by spark plasma sintering. J. Biomed. Mater. Res. Part A 2012, 101A, 1489–1501.
- Kim, H.W.; Georgiou, G.; Knowles, J.C.; Koh, Y.H.; Kim, H.E. Calcium phosphates and glass composite coatings on zirconia for en-hanced biocompatibility. Biomaterials 2004, 25, 4203–4213.
- Yazdani, J.; Ahmadian, E.; Sharifi, S.; Shahi, S.; Dizaj, S.M. A short view on nanohydroxyapatite as coating of dental implants. Biomed. Pharmacother. 2018, 105, 553–557.
- Barrè Re, F.; van der Valk, C.M.; Meijer, G.; Dalmeijer, R.A.J.; de Groot, K.; Layrolle, P. Osteointegration of Biomimetic Apatite Coating Applied onto Dense and Porous Metal Implants in Femurs of Goats. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 67, 655–665.
- Habibovic, P.; Li, J.; van der Valk, C.M.; Meijer, G.; Layrolle, P.; van Blitterswijk, C.; de Groot, K. Biological performance of uncoated and octacalcium phosphate-coated Ti6Al4V. Biomaterials 2005, 26, 23–36.
- Rocci, A.; Rocci, M.; Rocci, C.; Scoccia, A.; Gargari, M.; Martignoni, M.; Gottlow, J.; Sennerby, L. Immediate loading of Brånemark system TiUnite and machined-surface implants in the posterior mandible, part II: A randomized open-ended 9-year follow-up clinical trial. Int. J. Oral Maxillofac. Implant. 2013, 28, 891–895.
- Traini, T.; Murmura, G.; Sinjari, B.; Perfetti, G.; Scarano, A.; D’Arcangelo, C.; Caputi, S. The Surface Anodization of Titanium Dental Implants Improves Blood Clot Formation Followed by Osseointegration. Coatings 2018, 8, 252.
- Shalabi, M.M.; Gortemaker, A.; Van’t Hof, M.V.; Jansen, J.A.; Creugers, N.H.J. Implant Surface Roughness and Bone Healing: A Systematic Review. J. Dent. Res. 2006, 85, 496–500.
- Sreeharsha, T.; Sharan, S.; Chandra, P.K.; Badola, I.; Jabeen, N.S. Implant surface modifications: A review. Int. J. Appl. Dent. Sci. 2020, 6, 334–338.
- Al-Nawas, B.; Groetz, K.A.; Goetz, H.; Duschner, H.; Wagner, W. Comparative histomorphometry and resonance frequency analysis of implants with moderately rough surfaces in a loaded animal model. Clin. Oral Implant. Res. 2007, 19, 1–8.
- Cho, S.A.; Park, K.T. The removal torque of titanium screw inserted in rabbit tibia treated by dual acid etching. Biomaterials 2003, 24, 3611–3617.
- Grassi, S.; Piattelli, A.; De Figueiredo, L.C.; Feres, M.; De Melo, L.; Iezzi, G.; Alba, R.C.; Shibli, J.A. Histologic Evaluation of Early Human Bone Response to Different Implant Surfaces. J. Periodontol. 2006, 77, 1736–1743.
- Hirano, T.; Sasaki, H.; Honma, S.; Furuya, Y.; Miura, T.; Yajima, Y.; Yoshinari, M. Proliferation and osteogenic differentiation of human mesenchymal stem cells on zirconia and titanium with different surface topography. Dent. Mater. J. 2015, 34, 872–880.
- Kim, H.-K.; Woo, K.M.; Shon, W.-J.; Ahn, J.-S.; Cha, S.; Park, Y.-S. Comparison of peri-implant bone formation around injection-molded and machined surface zirconia implants in rabbit tibiae. Dent. Mater. J. 2015, 34, 508–515.
