Cushing’s syndrome is characterized by an exogenous or endogenous excess of glucocorticoids (GCs) resulting in a combination of metabolic disorders, including visceral obesity, type 2 diabetes mellitus, dyslipidaemia and cardiovascular disease. Nutrition has an important role in the management of obesity, diabetes mellitus and cardiovascular disease and may be used as an additional treatment of the metabolic comorbidities in patients with CS waiting to undergo neurosurgery or to reach pharmacologically biochemical remission.
Traditional methods of weight loss include various types of low-calorie diets calculated on a calorie range of between 800 and 1500 Kcal per day, although calorie requirements vary from individual to individual, so the goal of weight loss can also be achieved with a higher calorie amount [
1]. A VLCKD is a nutritional approach characterized by low daily caloric intake (less than 800 kcal/day), low carbohydrate (<50 g/day) and normoproteic (1–1.5 g of protein/kg of ideal body weight) contents [
2,
3]. This dietogenic protocol leads to the production of ketones, which are then used by other tissues such as the central nervous system, skeletal muscle and heart for energy production [
4].
A VLCKD has been reported to induce a significant weight loss and improvement in lipid parameters, glycaemic indices and insulin sensitivity, beyond an improvement in neurological and respiratory disorders [
5,
6,
7,
8,
9,
10].
2. Cushing’s Syndrome
CS is characterized by an excess of GCs that can be exogenous due to a chronic intake of corticosteroids or endogenous owing to the pituitary or adrenal hyperproduction of ACTH or cortisol, respectively. Rarely, the endogenous form can result from an extra-pituitary ACTH-secreting tumour (ectopic CS) [
11].
CS is associated with increased mortality and a high risk of cardiovascular disease due to the presence of several comorbidities [
12,
13,
14,
15,
16]. Comorbidities of CS include metabolic syndrome, characterized by systemic arterial hypertension, visceral obesity, impaired glucose metabolism and dyslipidaemia, polycystic ovary syndrome (PCOS), musculoskeletal disorders, such as myopathy, osteoporosis and skeletal fractures, infections, neuropsychiatric disorders, such as impaired cognitive function, depression or mania, impaired reproductive and sexual function and dermatological manifestations, represented mainly by acne, hirsutism and alopecia [
14,
15,
16,
17,
18].
The therapeutic approach consists of surgery as the first-line therapy. When patients refuse surgery or it is contraindicated or when a relapse occurs, other therapeutic options including medical therapy, radiotherapy or bilateral adrenalectomy should be evaluated [
19,
20]. Medical therapy mainly consists of drugs directly inhibiting pituitary ACTH secretion, such as pasireotide and cabergoline, or adrenal steroidogenesis inhibitors such as metyrapone, ketoconazole, osilodrostat, mitotane and etomidate [
21,
22]. Another medical drug is the glucocorticoid receptor (GR) antagonist mifepristone, which impairs cortisol binding to GR and mainly acts on clinical comorbidities [
23]. However, obtaining remission in CS is not always possible and sometimes it is necessary to combine the treatment options. In addition, these patients tend to show the metabolic comorbidities for a long time, sometimes even in the remission phase, and therefore a nutritional approach to improve metabolism should be started.
Nutrition and Cushing’s Syndrome
Generally speaking, patients with CS need a low sodium, high-protein and high-calcium diet to prevent muscle and bone loss, respectively. However, an interesting and complex relationship exists between diet macronutrients and cortisol [
24].
Meal macronutrients have a strong influence on cortisol concentrations, reducing or increasing their levels [
25,
26,
27,
28]. Long-standing studies have shown that fasting and starvation are associated with an increase in serum, salivary and urinary free cortisol levels and inadequate suppression of cortisol after a low dose dexamethasone test [
29,
30,
31,
32]. However, to what degree caloric restriction cortisol levels increased was not ascertained in any study [
33,
34].
The sympathetic nervous system (SNS) and hypothalamic–pituitary–adrenal axis (HPA) are strictly involved in stress management. An unhealthy behaviour (consumption of highly rich carbohydrate food, chronic stress and reduced sleep) may stimulate cortisol secretion with the development of obesity in subjects who are predisposed for it [
35]. By contrast, calorie-restricted diets inducing a decrease in cortisol values are associated with weight loss and reduced chronic inflammation [
36].
There are a few clinical studies evaluating the effects of calorie restriction on cortisol levels.
Stimson et al. evaluated the effects of a high-fat/low-carb diet vs. a moderate-fat/moderate-carb diet on cortisol metabolism in obese men [
37]. They showed that a lower carb diet was able to regenerate cortisol by increasing the enzyme 11-β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) that activates cortisol and reducing the enzymes (5-alpha and 5-beta reductase) that inactivate cortisol. Other improvements seen in the lower carb group were greater weight loss and improvements in glucose and insulin levels. The regeneration of cortisol in the low-carb group was independent of the difference in caloric intake between the low-carb and the high-carb group, meaning that the number of calories consumed was not a factor in the positive changes seen; rather, it was the carb ratio in the diet that made the difference.
Another study by the same authors showed that dietary macronutrients have different effects on cortisol production [
38]. They conducted a study on eight lean men and observed the effects of carbohydrate, high-protein and fat meals on insulin and cortisol levels, showing that all these meals stimulate a rise in cortisol levels, in different ways. Indeed, carbohydrates stimulate both the adrenal cortisol secretion and the extra-adrenal cortisol regeneration mediated by 11β-HSD1, which is present in the liver, adipose tissue and brain, and regenerate cortisol from cortisone releasing it into the bloodstream. By contrast, high-protein and fat meals stimulate adrenal cortisol secretion to a greater degree than extra-adrenal regeneration. The extra-adrenal cortisol regeneration is strictly related to the increase in insulin levels.
Interestingly, a meta-analysis conducted on 13 studies analysed the effects of fasting, a very low-calorie diet (VLCD) and a low-calorie diet (LCD) on serum cortisol levels [
39]. This meta-analysis only included studies that evaluated serum cortisol levels, while it excluded those based on salivary or urinary cortisol levels, in order to avoid heterogeneity of the studies. The results of the meta-analysis showed that short-term calorie restriction was more associated with an increase in cortisol values, compared to a VLCD and LCD, which, in turn, had no long-term effects on serum cortisol values and were less stressful than fasting. In addition, carbohydrate restriction was associated with a decrease in insulin concentration, leading to extra-adrenal cortisol synthesis [
39].
Furthermore, some amino acids, such as tryptophan, can lead to a decrease in cortisol levels [
40]. Similarly, supplementation with phospholipids at the dose of at least 400 mg/day also results in a reduction in cortisol levels [
41]. In addition, other nutrients including vitamin B6 and B12, folic acid, lithium, taurine, fermented milk products and sprouts of brown rice, barley and beans, which stimulate the GABAergic system, in turn, can reduce the secretion of CRH, leading to a decrease in cortisol levels [
24,
41,
42,
43].
3. Ketogenic Diet
The ketogenic diet (KD) was used for the first time for the treatment of epilepsy in 1921 [
44]. Its use was proposed to mimic the effects of fasting. The KD is a high-fat, low carbohydrate, normocaloric diet. It is characterized by a 4:1 ratio of fat to protein, plus carbohydrates and about 90% of calories are provided by fats (
Table 1). In the scenario of the use of a KD, some variants can be identified, the low-calorie ketogenic diet (LCKD) with a calorie intake of 800–1200 Kcal/day and the very-low-calorie ketogenic diet (VLCKD) with a calorie intake of less than 800 Kcal/day (
Table 1) [
45].
Table 1. Characteristics of classical ketogenic diet (KD), low-calorie ketogenic diet (LCKD) and very low-calorie ketogenic diet (VLCKD).
| |
KD
|
LCKD
|
VLCKD
|
|
Caloric intake
|
Normocaloric
|
800–1200 Kcal/day
|
<800 Kcal/day
|
|
Carbohydrate (%)
|
5–10
|
13
|
13
|
|
Protein (%)
|
15–20
|
29
|
44
|
|
Fat (%)
|
70–80
|
58
|
43
|
|
Foods
|
Vegetable oils, fish, eggs, meat, cheese, olives, avocado, coconut
|
Natural high biological value proteins (1–2 servings) including meat, fish, eggs, processed meat
|
Replacement meals with high biological value proteins composed by 18 g of proteins, 4 g of carbohydrates and 3 g of fats
|
|
Recommendations for use [1]
|
-
Epilepsy and resistance to antiepileptic therapy
-
Gliomas and glioblastomas
-
Neurodegenerative diseases (Alzheimer or Parkinson’s disease)
-
Neurocognitive disorders
-
Brain trauma
|
-
Obesity BMI 25–35 kg/m2
-
Obesity associated with arterial hypertension or type 2 diabetes mellitus or hypertriglyceridaemia or heart failure or polycystic ovary syndrome
-
Paediatric obesity with epilepsy and/or insulin resistance
|
-
Severe obesity
-
Obesity complicated by type 2 diabetes or arterial hypertension or hypertriglyceridaemia or metabolic syndrome or OSAS or arthropathies
-
Obesity with indication of bariatric surgery
-
Adolescents with severe obesity
|
4. VLCKD
A VLCKD is characterized by, approximately, a 44–43–13% ratio of lipids, proteins and carbohydrates, respectively, and the total energy intake is less than or equal to 800 kcal [
55,
56,
57,
58,
59,
60].
In a VLCKD, the process of using ketone bodies as an energy source derived from fatty acids is much more intense, with increased use of these energy sources by tissues such as the heart, kidney, skeletal muscle and central nervous system. Physiologically, acetyl-CoA fuses with oxaloacetate derived from glycolytic processes. Under conditions of slowed glycolysis, such as during a VLCKD, the oxaloacetate produced is preferentially used for neoglucogenetic processes, while the cetyl-CoA molecules derived from the beta-oxidation of fatty acids are used for the production of ketone bodies. The VLCKD is the model with the greatest availability of acetyl-CoA [
61,
62].
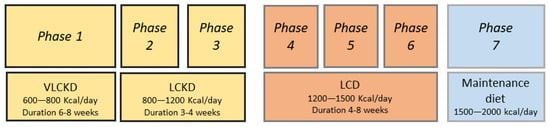
A VLCKD plan is normally divided into several phases, with an initial pure ketogenic period of 6–8 weeks (Figure 1).
Figure 1. Phases of very low-calorie ketogenic diet (VLCKD) protocol. LCKD: low-calorie ketogenic diet. LCD: low carbohydrate diet.
Protein preparations containing 18 g of proteins, 4 g of carbohydrates and 3 g of fats may be used initially, but these are gradually discontinued with the introduction of natural protein foods. In phase 1 of the VLCKD, patients are educated to eat high biological value protein preparations five times a day and vegetables with a low glycaemic index. In phase 2, natural proteins including meat/egg/fish are introduced in place of one of the protein preparations at lunch or dinner. In phase 3, natural proteins are introduced in place of the second protein preparation. At the end of the VLCKD, carbohydrates are gradually reintroduced, starting with foods with a lower glycaemic index including fruit and milk products (Phase 4), followed by foods with a moderate glycaemic index such as legumes (Phase 5) and a high glycaemic index (bread, pasta and cereals—Phase 6). This dietetic plan corresponds to an LCD with a daily calorie intake ranging from 1200 to 1500 Kcal/day. At the end of phases 4–6, the patient must be re-educated in order to be able to have a maintenance diet of approximately 1500–2000 Kcal/day and avoid regaining lost weight [
58,
63].
In a VLCKD, insulin levels are reduced, while glucagon levels increase, and after a few days, circulating levels of free fatty acids and ketone bodies rise. The success of a VLCKD depends not only on the anorectic power of ketone bodies but also on the contribution of certain hormones produced in higher concentrations, such as neuropeptide Y, cholecystokinin and ghrelin. In addition, carbohydrate reduction leads to the rapid consumption of hepatic triglycerides and increased intrahepatic beta-oxidation [
64].