Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The unique biology of flies and their omnipresence in the environment of people and animals makes them ideal candidates to be important vectors of antimicrobial resistance genes. Consequently, there has been increasing research on the bacteria and antimicrobial resistance genes that are carried by flies and their role in the spread of resistance.
- flies
- bacterial pathogens
- antimicrobial-resistance
- sentinel
- surveillance
1. Introduction
Flies are insects in the order Diptera that have one pair of wings for flight and a residual second pair of wings, known as knobs, which are used for balance [1]. Over 125,000 species of Dipterans have been identified, including gnats, midges, mosquitoes, leaf miners, horse flies, houseflies, blowflies, and fruit flies. Houseflies, Musca domestica Linnaeus (Diptera: Muscidae), are of particular importance as they are notorious “pests” that can transmit a variety of bacterial pathogens [2]. They are thought to have originated in the savannahs of Central Asia and later spread worldwide [3], particularly in tropical and subtropical areas where they are mostly associated with people and domestic animals in both rural and urban areas [4].
Flies have four life stages—eggs, larvae/maggots, pupae, and adults [5]. Female houseflies lay their eggs in compost, trash, soiled bedding, or manure containing moist and microbial-rich decaying organic matter near people’s houses and farms [2]. Each female can oviposit four to six times in her lifetime, each time producing 100–150 eggs [2][6]. The eggs usually hatch within 8–12 h if the environment is moist and at an optimal temperature of 25 °C to 30 °C [2]. The first-instar larvae feed on bacteria in nutrient-rich environments and pass through a further two instars before becoming larvae/maggots that migrate into a dark, dry, and cool place where they pupate [2][6]. Adult flies emerge around 2–4 days later when ambient temperatures are 32 °C to 37 °C, meaning the entire life cycle of houseflies is very rapid, ranging from 10–21 days [7].
The behavior of houseflies promotes their ability to transmit bacterial pathogens [8][9]. They live in close proximity to people (synanthropic) or in their dwellings (endophilic cosmopolitan), and they often feed on animal and human feces (coprophagic) and decaying matter, such as garbage [7]. All life stages can thus be exposed to a variety of pathogens in unsanitary environments, and these can then be mechanically transmitted to people [10]. Adult flies can move over distances of up to 20 miles in their lifetime, which means they are ubiquitous in the environment and well capable of disseminating pathogens from unsanitary areas into people’s homes and places of work and leisure [11].
2. Flies as Vectors of Bacterial Pathogens
Flies can carry a surprising diversity and number of pathogens. One systemic review revealed more than 130 human pathogens have been identified in houseflies [3], including bacteria, fungi, viruses, and parasites [3][12] The predominant pathogens are bacteria, including Klebsiella spp. [13], Salmonella [14], Pseudomonas aeruginosa [15], Campylobacter jejuni [16], Edwardisella spp. [17], Clostridium spp. [18], Yersinia enterocolitica [19], and Burkholderia pseudomalliei [20]. Recently, Balaraman et al. reported that houseflies acquired and harbored infectious SARS-CoV-2 for up to 24 h post-exposure. They could mechanically transmit SARS-CoV-2 genomic RNA to the surrounding environment for up to 24 h post-exposure [12].
2.1. External Carriage of Bacterial Pathogens
Flies have unique body structures that enable them to effectively carry bacteria. For example, bacteria readily become attached to the sticky leg pads, hairs, electrostatically charged exoskeleton, and sponging mouthparts of flies [8][21][22]. A study quantifying the transfer of fluorescence-labeled E. coli from sugar, milk, steak, and potato salad to houseflies revealed a single housefly can carry up to 2 × 1012 E. coli and approximately 0.1 mg of food between landing sites [23]. Flies were also found to externally carry Enterococcus faecium in poultry farms [24], Klebsiella pneumoniae in kitchens and farms [25], Salmonella enterica in swine farms [26], and Staphylococcus aureus in urban areas [19]. Female flies carry more bacteria than males because they visit oviposition sites that are heavily contaminated with bacteria [27].
2.2. Internal Carriage of Bacterial Pathogens
Although most studies have focused on bacteria carried on the body surface, some researchers have investigated bacteria carried internally within the digestive tract [28][29][30][31] Ingested material containing bacteria is initially stored in the crop, from where it passes down the digestive tract through the proventriculus/foregut, midgut, hindgut, and rectum [32]. Whereas epithelial cells in the foregut and hindgut are covered with a protective cuticle [33], the midgut is lined with a unique structure, named the “peritorphic matrix”, peritrophic envelope, or peritrophic membrane (PM) [29][34] (Figure 1). This is a double-layered, noncellular structure composed of chitin, proteoglycans, and various proteins [33] that serves as a physical barrier to prevent microbes in the ingesta from invading epithelial cells and causing damage [34][35]. The PM has gaps, ranging from 2 to 10 nm, which enable digestive enzymes, acid, and secretions to enter the endoperitrophic space and digest food materials [34][36]. At the same time, as part of an innate immune response, antimicrobial peptides, reactive oxygen species, and other epithelial secretions can enter the lumen and kill and digest the trapped bacteria [34]. Not all bacteria are killed, however, with species, such as Pseudomonas aeruginosa [28], Salmonella enterica serovar Typhimurium [29], and Aeromonas caviae [30][31], being able to be ingested and proliferate in the midgut before being shed in the feces in high numbers. The survival rate of ingested bacteria is dose-dependent [37] and also dependent on competition with the commensal microbiota [38]. Studies have shown that the numbers of pathogens in the digestive tract are three times higher than on the body surface, probably due to the multiplication of the pathogens in the digestive tract [39][40][41].
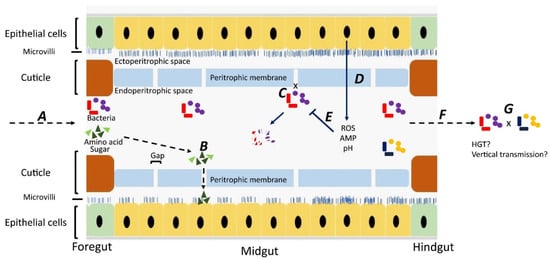
Figure 1. Fate of bacteria in the digestive tract (midgut) of flies. A: Ingested food with bacteria is predigested with saliva in the crop. The epithelial cells in the foregut are covered by a cuticle, which prevents bacterial invasion. B: In the midgut, digestion products can pass through gaps in the peritrophic membrane and enter the ectoperitrophic space to be absorbed by epithelial cells. C: Bacteria cannot pass through the gaps on the peritrophic membrane and remain in the endoperitrophic space. D: Bacteria are trapped in the endoperitrophic space, triggering an innate immune response in epithelial cells to produce reactive oxygen species (ROS) and antimicrobial peptides (AMP). E: Trapped bacteria are killed by ROS, AMP, pH changes, and digestive enzymes. F: Some bacteria survive in the hostile environment, pass through to the hindgut, and are shed. G: There might be horizontal gene transfer between bacteria surviving in the digestive tract and bacteria may be transmitted vertically to offspring.
This entry is adapted from the peer-reviewed paper 10.3390/vetsci9060300
References
- Yarger, A.M.; Fox, J.L. Dipteran halteres: Perspectives on function and integration for a unique sensory organ. Integr. Comp. Biol. 2016, 56, 865–876.
- Nayduch, D.; Burrus, R.G. Flourishing in filth: House fly—Microbe interactions across life history. Ann. Entomol. Soc. Am. 2017, 110, 6–18.
- Khamesipour, F.; Lankarani, K.B.; Honarvar, B.; Kwenti, T.E. A systematic review of human pathogens carried by the housefly (Musca domestica L.). BMC Public Health 2018, 18, 1049.
- Ommi, D. Molecular detection and antimicrobial resistance of Aeromonas from houseflies (Musca domestica) in Iran. Rev. MVZ Córdoba 2015, 20, 4929–4936.
- de Jonge, N.; Michaelsen, T.Y.; Ejbye-Ernst, R.; Jensen, A.; Nielsen, M.E.; Bahrndorff, S.; Nielsen, J.L. Housefly (Musca domestica L.) associated microbiota across different life stages. Sci Rep. 2020, 10, 7842.
- Cortinhas, L.B.; Martins Mendonça, P.; Braga, M.V.; Queiroz, M.M.C. Ultrastructure of the immature stages of Musca domestica (Diptera: Muscidae: Muscinae). J. Med. Entomol. 2020, 57, 1712–1721.
- Stafford, K. Fly Management Handbook: A Guide to Biology, Dispersal, and Management of the House Fly and Related Flies for Farmers, Municipalities, and Public Health Officials; The Connecticut Agricultural Experiment Station: New Haven, CT, USA, 2008.
- Onwugamba, F.C.; Fitzgerald, J.R.; Rochon, K.; Guardabassi, L.; Alabi, A.; Kühne, S.; Grobusch, M.P.; Schaumburg, F. The role of filth ‘flies’ in the spread of antimicrobial resistance. Travel Med. Infect. Dis. 2018, 22, 8–17.
- Förster, M.; Klimpel, S.; Mehlhorn, H.; Sievert, K.; Messler, S.; Pfeffer, K. Pilot study on synanthropic flies (e.g. Musca, Sarcophaga, Calliphora, Fannia, Lucilia, Stomoxys) as vectors of pathogenic microorganisms. Parasitol. Res. 2007, 101, 243–246.
- Chaiwong, T.; Srivoramas, T.; Sukontason, K.; Sanford, M.R.; Moophayak, K.; Sukontason, K.L. Survey of the synanthropic flies associated with human habitations in ubon ratchathani province of northeast Thailand. J. Parasitol. Res. 2012, 2012, 613132.
- Murvosh, C.M.; Thaggard, C.W. Ecological studies of the house fly. Ann. Entomol. Soc. Am. 1966, 59, 533–547.
- Balaraman, V.; Drolet, B.S.; Mitzel, D.N.; Wilson, W.C.; Owens, J.; Gaudreault, N.N.; Meekins, D.A.; Bold, D.; Trujillo, J.D.; Noronha, L.E.; et al. Mechanical transmission of SARS-CoV-2 by house flies. Parasites Vectors 2021, 14, 214.
- Fotedar, R.; Banerjee, U.; Samantray, S.J.C. Vector potential of hospital houseflies with special reference to Klebsiella species. Epidemiol. Infect. 1992, 109, 143–147.
- Mawak, J.D.; Olukose, O.J. Vector potential of houseflies (Musca domestica) for pathogenic organisms in Jos, Nigeria. J. Pest Dis. Vector Manag. 2006, 7, 418–423.
- Hemmatinezhad, B.; Ommi, D.; Hafshejani, T.T.; Khamesipour, F. Molecular detection and antimicrobial resistance of Pseudomonas aeruginosa from houseflies (Musca domestica) in Iran. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 18.
- Gill, C.; Bahrndorff, S.; Lowenberger, C. Campylobacter jejuni in Musca domestica: An examination of survival and transmission potential in light of the innate immune responses of the house flies. Insect Sci. 2017, 24, 584–598.
- Satish, S.; Saksham, C.; Ther, S.V.; Rakesh, S.; Ravi, S.; Central Poultry Diagnostic Laboratory (Phoenix Group). Isolation and identification of enterobacterial species from Musca domestica in broiler farms of Madhya Pradesh. Vet. Pract. 2013, 14, 239–241.
- Bahrndorff, S.; de Jonge, N.; Skovgård, H.; Nielsen, J.L. Bacterial communities associated with houseflies (Musca domestica L.) sampled within and between farms. PLoS ONE 2017, 12, e0169753.
- Rahuma, N.; Ghenghesh, K.S.; Ben Aissa, R.; Elamaari, A. Carriage by the housefly (Musca domestica) of multiple-antibiotic-resistant bacteria that are potentially pathogenic to humans, in hospital and other urban environments in Misurata, Libya. Ann. Trop. Med. Parasitol. 2005, 99, 795–802.
- Sulaiman, S.; Othman, M.Z.; Aziz, A.H. Isolations of enteric pathogens from synanthropic flies trapped in downtown Kuala Lumpur. J. Vector Ecol. 2000, 25, 90–93.
- Graczyk, T.K.; Knight, R.; Gilman, R.H.; Cranfield, M.R. The role of non-biting flies in the epidemiology of human infectious diseases. Microbes Infect. 2001, 3, 231–235.
- Wiktorczyk-Kapischke, N.; Skowron, K.; Kwiecińska-Piróg, J.; Białucha, A.; Wałecka-Zacharska, E.; Grudlewska-Buda, K.; Kraszewska, Z.; Gospodarek-Komkowska, E. Flies as a potential vector of selected alert pathogens in a hospital environment. Int. J. Environ. Health Res. 2021, 1–20.
- De Jesús, A.J.; Olsen, A.R.; Bryce, J.R.; Whiting, R.C. Quantitative contamination and transfer of Escherichia coli from foods by houseflies, Musca domestica L. (Diptera: Muscidae). Int. J. Food Microbiol. 2004, 93, 259–262.
- Graham, J.P.; Price, L.B.; Evans, S.L.; Graczyk, T.K.; Silbergeld, E.K. Antibiotic resistant enterococci and staphylococci isolated from flies collected near confined poultry feeding operations. Sci. Total Environ. 2009, 407, 2701–2710.
- Ranjbar, R.; Izadi, M.; Hafshejani, T.T.; Khamesipour, F. Molecular detection and antimicrobial resistance of Klebsiella pneumoniae from house flies (Musca domestica) in kitchens, farms, hospitals and slaughterhouses. J. Infect. Public Health 2016, 9, 499–505.
- Wang, Y.-C.; Chang, Y.-C.; Chuang, H.-L.; Chiu, C.-C.; Yeh, K.-S.; Chang, C.-C.; Hsuan, S.-L.; Lin, W.-H.; Chen, T.-H. Transmission of Salmonella between swine farms by the housefly (Musca domestica). J. Food Prot. 2011, 74, 1012–1016.
- Neupane, S.; White, K.; Thomson, J.L.; Zurek, L.; Nayduch, D. Environmental and sex effects on bacterial carriage by adult house flies (Musca domestica L.). Insects 2020, 11, 401.
- Joyner, C.; Mills, M.K.; Nayduch, D. Pseudomonas aeruginosa in Musca domestica L.: Temporospatial examination of bacteria population dynamics and house fly antimicrobial responses. PLoS ONE 2013, 8, e79224.
- Chifanzwa, R.; Nayduch, D. Dose-dependent effects on replication and persistence of Salmonella enterica serovar Typhimurium in house flies (Diptera: Muscidae). J. Med. Entomol. 2018, 55, 225–229.
- Nayduch, D.; Honko, A.; Noblet, G.P.; Stutzenberger, F. Detection of Aeromonas caviae in the common housefly Musca domestica by culture and polymerase chain reaction. Epidemiol. Infect. 2001, 127, 561–566.
- Nayduch, D.; Noblet, G.P.; Stutzenberger, F.J. Vector potential of houseflies for the bacterium Aeromonas caviae. Med. Vet. Entomol. 2002, 16, 193–198.
- Doud, C.W.; Zurek, L. Enterococcus faecalis OG1RF:pMV158 survives and proliferates in the house fly digestive tract. J. Med. Entomol. 2012, 49, 150–155.
- Kelkenberg, M.; Odman-Naresh, J.; Muthukrishnan, S.; Merzendorfer, H. Chitin is a necessary component to maintain the barrier function of the peritrophic matrix in the insect midgut. Insect Biochem. Mol. Biol. 2015, 56, 21–28.
- Lemaitre, B.; Miguel-Aliaga, I. The digestive tract of Drosophila melanogaster. Annu. Rev. Genet. 2013, 47, 377–404.
- Lehane, M.J.; Msangi, A.R. Lectin and peritrophic membrane development in the gut of Glossina m.morsitans and a discussion of their role in protecting the fly against trypanosome infection. Med. Vet. Entomol. 1991, 5, 495–501.
- Lehane, M.J. Peritrophic matrix structure and function. Annu. Rev. Entomol. 1997, 42, 525–550.
- Kumar, N.H.; Nayduch, D. Dose-dependent fate of GFP-expressing Escherichia coli in the alimentary canal of adult house flies. Med. Vet. Entomol. 2016, 30, 218–228.
- Greenberg, B.; Kowalski, J.A.; Klowden, M.J. Factors affecting the transmission of salmonella by flies: Natural resistance to colonization and bacterial interference. Infect. Immun. 1970, 2, 800–809.
- Rochon, K.; Lysyk, T.J.; Selinger, L.B. Retention of Escherichia coli by house fly and stable fly (Diptera: Muscidae) during pupal metamorphosis and eclosion. J. Med. Entomol. 2005, 42, 397–403.
- Mramba, F.; Broce, A.B.; Zurek, L. Vector competence of stable flies, Stomoxys calcitrans L. (Diptera: Muscidae), for Enterobacter sakazakii. J. Vector Ecol. 2007, 32, 134–139.
- Pava-Ripoll, M.; Pearson, R.E.; Miller, A.K.; Ziobro, G.C. Prevalence and relative risk of Cronobacter spp., Salmonella spp., and Listeria monocytogenes associated with the body surfaces and guts of individual filth flies. Appl. Environ. Microbiol. 2012, 78, 7891–7902.
This entry is offline, you can click here to edit this entry!
