Silicate materials can exist in nature in amorphous and crystalline states, for example, as inorganic glass and cements, which allows to call these materials antagonistic. They are characterized by a similar chemical composition but different quantitative contents .
Various methods have been proposed for controlling the alkaline activity of glass based on various types of alkaline extraction.
To control the alkaline activity of the glass surface, the phenomenon of high-temperature extraction can be used. As is known, in the high-temperature zone of a gas flame, a partially ionized gaseous medium is formed—a low-temperature isothermal plasma
[1]. At a stable flow rate of gas and air at a constant ratio, plasma is characterized by a certain degree of ionization α, and its electrical conductivity is a certain value of the magnitude of the current, Ipl. In the process of heating the glass surface with a gas flame, at a certain temperature—let's call it the temperature of the beginning of the extraction process, Tne—alkaline ions begin to evolve from the glass. When they enter the plasma flow, they sharply increase the electrical conductivity of the plasma. Thus, by controlling the temperature on the glass surface and simultaneously measuring the magnitude of the plasma current, it is possible to determine this temperature Tne.
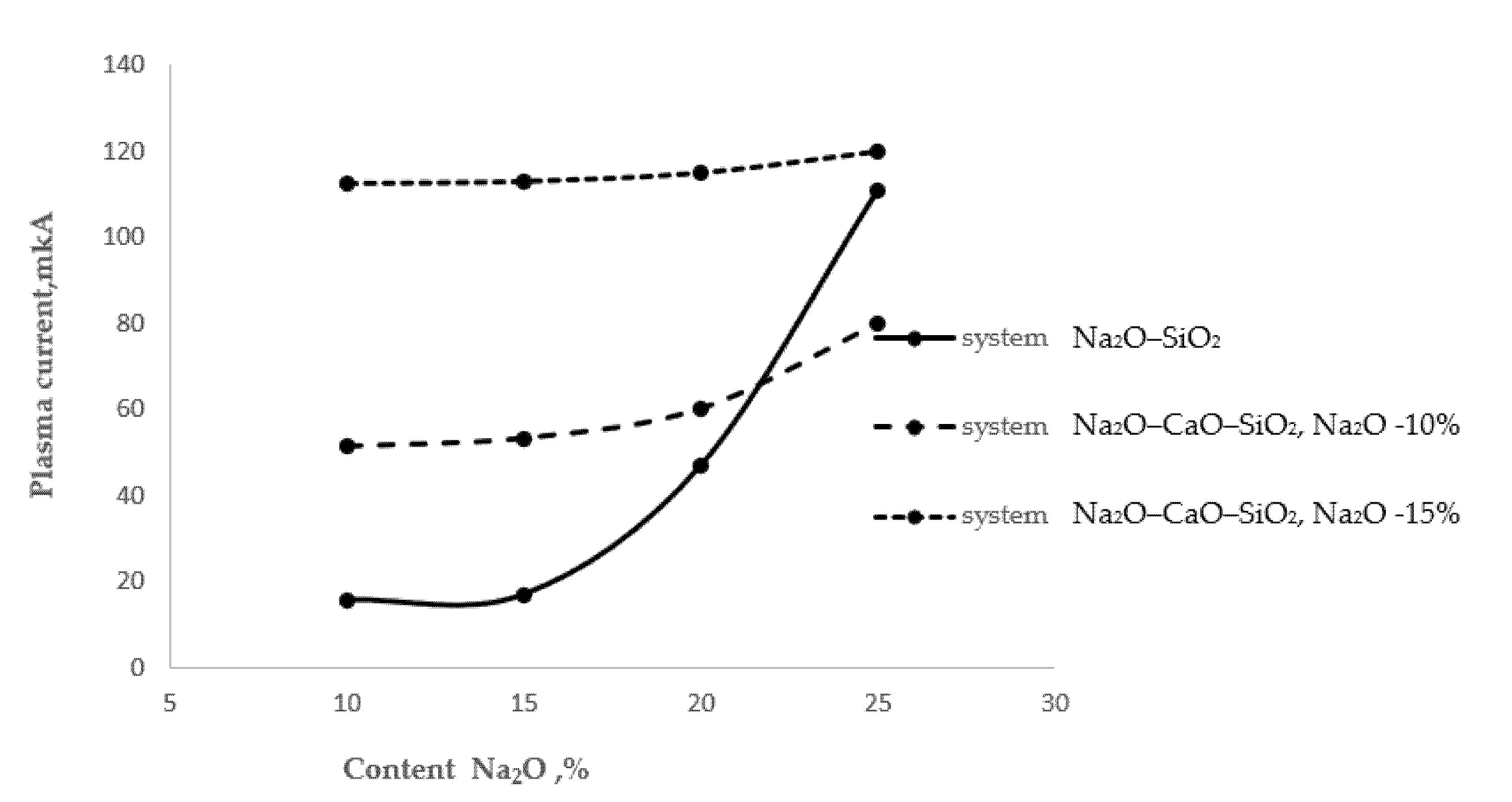
Figure 1 shows the process of extraction of alkaline cations from glasses with different surface alkali contents.
Figure 1. High-temperature extraction of alkali cations from various glasses.
As follows from Figure 1, as the alkali oxide content in the two-component glass of the Na2O–SiO2 system increased, the thermal extraction also increased. A particularly sharp increase in the amount of extracted sodium cations was observed in the glass with a 25% Na2O content. In the three-component glass of the Na2O–CaO–SiO2 system, at a stable Na2O content of 10% and with an increase in the CaO content from 10 to 20%, the plasma current increased by 17%, which may be associated with the extraction of sodium cations. In the same three-component glass with a stable Na2O content of 15%, the plasma current increased by 2.2%. An almost twofold increase in the plasma current should be noted in comparison with the 10% Na2O content glass, probably associated with the extraction of calcium cations.
In addition to the method described above for determining the alkaline activity of a glass surface, one can use the method of activated thermionic emission by measuring the electric potential of a medium saturated with alkali cations
[2]. As mentioned above, the temperature of the onset of emission (extraction, tne) primarily depends on the alkali content of the glass (
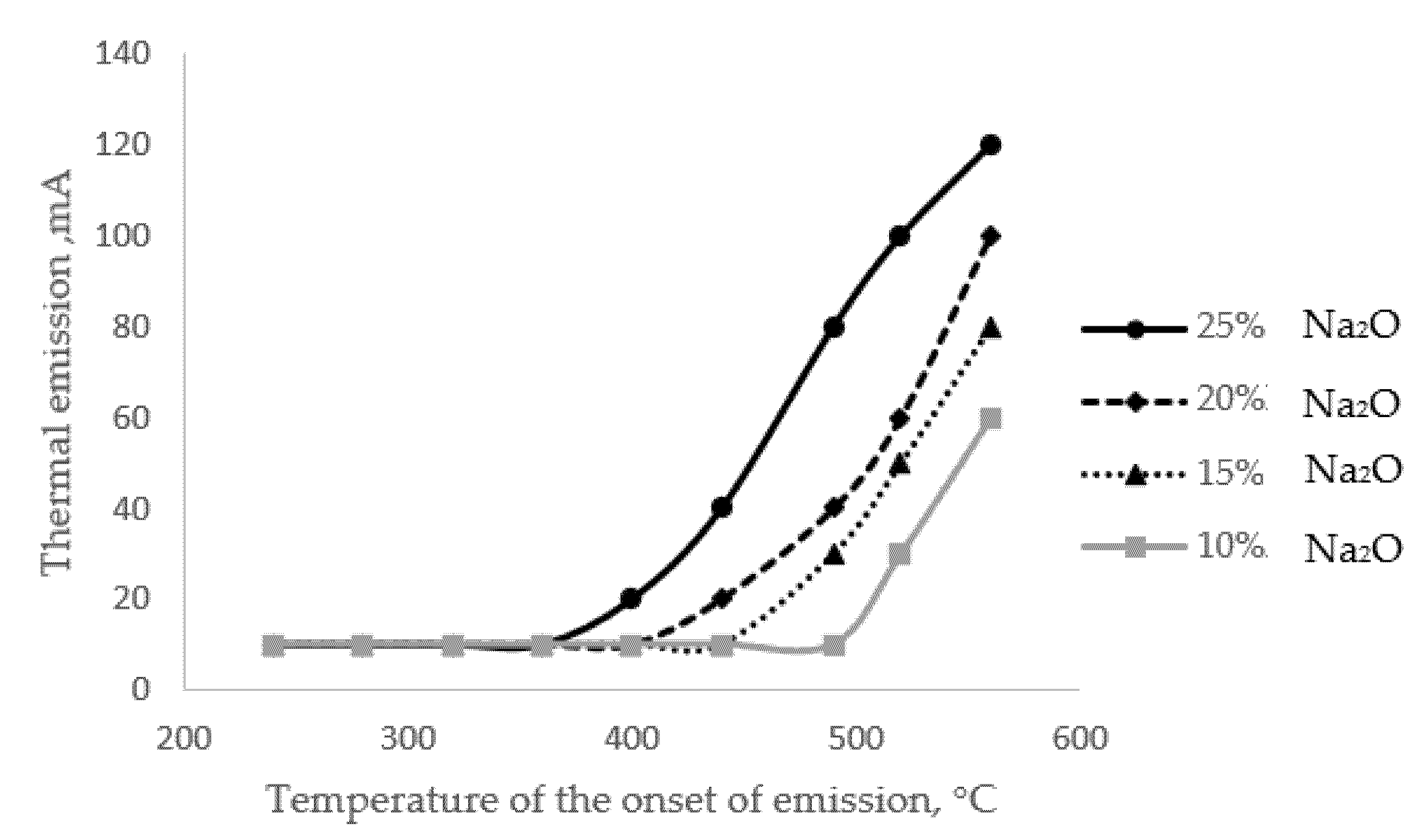
Figure 2).
Figure 2. Activated thermal emission curves for glasses with different alkali contents.
The results presented in Figure 2 show that there is a close relationship between the alkali content of the glass and the initial temperature of the extraction process; the correlation coefficient for this relationship is 0.9. The phenomenon of thermal emission itself is a special situation of the alkaline extraction process, which has a diffusion character.
The methods of high-temperature alkaline extraction also include thermochemical extraction
[3][4][5].
It is known
[6] that the presence of sulfur dioxide in the atmosphere surrounding glassware at temperatures close to the maximum upper annealing temperature leads to the interaction of the gas with the alkaline component of glass according to the scheme:
The amount of sodium sulfate formed depends on the temperature of the thermochemical treatment and its duration. With increasing temperature, the amount of sodium sulfate increases with a stable processing time. However, the amount of sodium sulfate formed on the glass surface at a constant temperature and duration will depend only on the alkali content on the glass surface. It should be noted that the mode of thermochemical treatment depends on the type of glass; for example, for window and container glass, it is 550 °C with a duration of 0.5–1 min
[7].
If it was assumed that the amount of extracted alkali is directly proportional to the concentration of alkali metal cations per unit volume of the surface layer of finite thickness, and the coefficient of diffusion of alkali metal cations through this layer, then the amount of extracted alkali can be calculated by using the formula:
where: Q is the amount of alkali extracted (mg/cm
2), Co is the concentration of sodium ions per unit volume of the surface layer of glass, D is the diffusion coefficient, and Ʈ is the duration of the thermochemical treatment (min).
In the situation of thermochemical extraction, the duration of the extraction process is almost 25 times higher than that for the standard method for determining water resistance, which can be explained by the higher diffusion coefficient
[8].
However, approximately the same amount of extracted alkalis Q
[9], at the level of 0.4–0.5 mg Na
2O/dm
2 in low-temperature ion-exchange and high-temperature thermochemical extraction methods, can be explained by different mechanisms of the extraction process. In
[4], it was found that the process of high-temperature extraction occurs in the kinetic region. This means that the overall rate of the extraction process is not limited by diffusion, as in the situation of ion-exchange extraction, but by the rate of reaction between sulfur dioxide and the alkaline component of the glass surface. In this situation, the factor limiting the overall rate of the extraction process is the deposition of reaction products on the glass surface. Thus, it can be assumed that the shorter the duration of the contact of the extractant with the glass surface, the better the amount of extracted cations will reflect their initial concentration on the surface. This condition is best met by the method for determining thermal extraction, according to which the duration of the extraction process is two orders of magnitude less than that in thermochemical methods and three orders of magnitude higher than that in ion-exchange method.
The extraction process, which is based on ion exchange, is the basis of the traditional method for determining the water resistance of glass
[6]. Upon contact with water, an ion exchange reaction takes place:
As a result of the action of water on the glass, its surface is hydrated, and the extractant turns from neutral to alkaline.
As is known, water in contact with glass at the initial stage acts selectively as a reagent of the first group, extracting only alkaline cations from glass
[10]. The latter, with OH
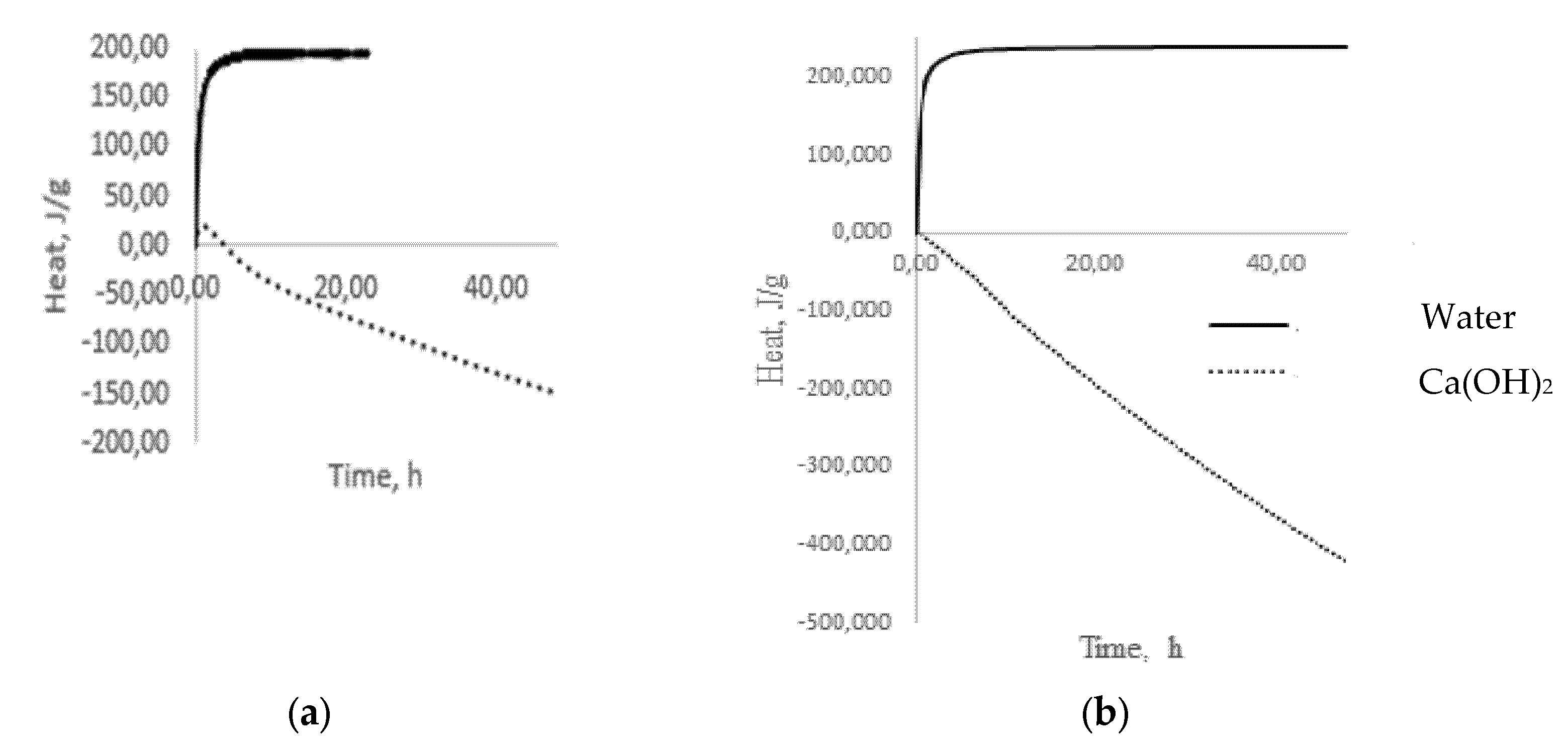
− groups of water, forms alkalis, which already belong to the reagents of the second group, completely dissolving glass. The results of microcalorimetric studies testify to the formation of alkalis (
Figure 3a)
[11]. The process of alkali formation proceeds most actively in the first 60 min from the moment of glass coming into contact with water, as evidenced by intense heat release (
Figure 3a), after which the stabilization period begins, indicating the completion of the selective extraction stage and the transition to the glass dissolution stage, accompanied by heat absorption (
Figure 3b).
Figure 3. Dependence of heat release on time when glass comes into contact with water and Ca(OH)2: (a) green glass; (b) low-alkali borosilicate glass.
A completely different picture emerges when glass is exposed to, for example, Ca(OH)2, which belongs to the reagents of the second group, which dissolve glass. During the first hour, there is a slight exo-effect (Figure 3), which can be associated with the formation of intermediate products and which decreases to zero over the next three hours. Then, the process of heat absorption is observed, which can be identified as a dissolution process (Figure 3). A similar picture forms in the situation of low-alkali borosilicate glass (Figure 3), with the only difference being that there is no exo-effect in the interaction with Ca(OH)2 in contrast to multi-alkali container glass. With prolonged contact of the glass with water at temperatures up to 100 °C, the concentration of the alkaline solution increases, and it begins to act on the glass as a reagent of the second group that dissolves glass. In this situation, the process of alkaline extraction can be conditionally divided into two stages: kinetic, in which only surface alkaline cations participate, and diffusion, in which alkaline cations from deeper layers of glass participate. The first of them is fast because of the low activation energies for the extraction of surface alkaline cations, and the second is slow because it is associated with diffusion. This separation of the extraction process into two stages is very important since, in some situations, it becomes necessary to assess the alkaline activity in short time intervals.
For example, when glass powder is used as an additive to Portland cement, alkaline cations of the first kinetic phase of extraction have a significant effect on the process of cement hydration in the pre-induction period of hydration; in parallel with alkaline extraction, the formation of hydrosilicate and calcium hydroxide occurs with a sharp increase in the pH of the medium
[12].
Under such conditions, the use of traditional methods for determining the water resistance of glass to assess its extraction ability is impossible due to the transition of the extractant from a neutral state to alkali, which allows to take part in the kinetic phase of the extraction process.
To assess the alkaline activity of the glass surface, the change in the pH of water at normal temperature (20 °C) after the introduction of a portion of the glass ca be used. In this siuation, the amount of extracted ions will depend on the specific surface area of the glass powder
[13].
Given the fact that, in accordance with the European standard EN 197-1, for cement composites in which part of the clinker cement is replaced by various hydraulic and pozzolanic additives, the factor of the total alkali content in such composites is of great importance, and the amount of alkali cannot exceed 2.0%
[14].
Currently, there are many works are devoted to the issue of using glass waste as additives to Portland cement
[15][16][17][18][19][20][21][22][23][24].
However, if talking about the possibility of using glass waste in a powdered state as an additive to Portland cement, it is necessary to take into account the contribution of the alkaline component of the glass to the total alkalinity of cement composites
[25]. This issue is discussed in detail in
[13]. In this situation, the ratio of the amount of alkaline ions on the glass surface to that in the volume is very important.
The extraction of alkali ions from the glass surface leads to the emergence of diffusion flows from deep layers due to the concentration gradient. It is extremely difficult to take into account their influence on alkaline activity; therefore, the minimum possible exposure time is a prerequisite for assessing the alkaline activity of powder materials. This approach makes it possible to calculate the alkaline activity from the alkali content of the glass. Until now, it was believed that the chemical stability of glass, as one of its few properties, could not be calculated. However, this becomes possible if it is assumed that the glass is chemically homogeneous and that the glass powder particles are cube-shaped. In this situation, the ratio of the amount of alkali ions on the surface to their content in the volume will be approximately 0.6 for a cube face size of 1 mm
2. In this situation, the alkaline activity will be equal to
[13]:
where Ac is the calculated value of the alkaline activity of the glass powder (mg Na
2Oeq/m
2), C is the total alkali content of the glass (mg Na
2Oeq/g glass), Ce is the amount of extracted Na
2Oeq (%), and S is the specific surface area of the glass powder (m
2/g).
The calculated alkaline activity values for the most common container glasses are 4.12, 3.84, and 4.54 mg Na
2Oeq mg/m
2 for colorless, brown, and green glass, respectively
[13].