Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Immunotherapy has redefined the treatment of cancer patients and it is constantly generating new advances and approaches. Among the multiple options of immunotherapy, bispecific antibodies (bsAbs) represent a novel thoughtful approach. These drugs integrate the action of the immune system in a strategy to redirect the activation of innate and adaptive immunity toward specific antigens and specific tumor locations.
- bispecific antibodies
- immunotherapy
- immune restoration
- cancer therapy
1. Introduction
Regardless of efforts from the scientific community, options to treat cancer patients in advanced stages achieving complete response with low recurrence are limited. For that reason, the search of effective alternatives to treat cancer has increase in the last years. Currently, some alternatives that are effective in the treatment of other conditions are now being studied as an alternative to treat cancer patients. Monoclonal antibodies are known to have a positive impact on many conditions such as autoimmune disorders, cardiovascular, pulmonary, and even infectious diseases [1]. Even though monoclonal antibodies are usually specific to one epitope, genetic and cell engineering have allowed the biosynthesis of bispecific antibodies (bsAbs). BsAbs were first described by Nisonoff et al. over 60 years ago; however, they gained clinical relevance after the first approval by the Food and Drugs Administration (FDA) [2] of blinatumomab, a bsAb approved for the treatment of acute myeloid leukemia. Since then, these molecules have become an attractive choice to treat cancer, due to their efficacy and safety profile (Figure 1) [3]. The original concept of bsAbs was a molecule that can bind to two different epitopes [2].
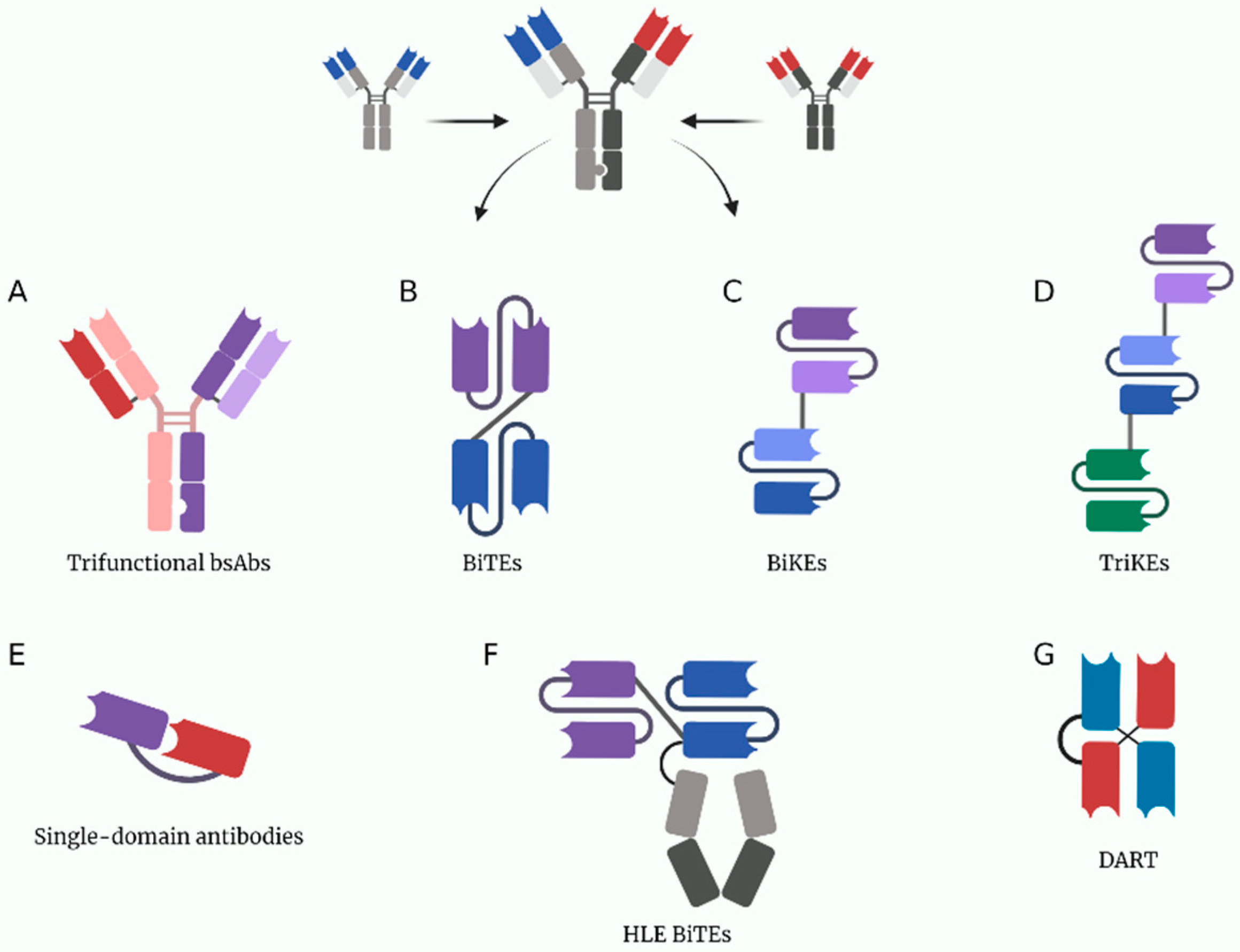
Figure 1. A depiction of some current multivalent antibody structures under study. (A) Trifuctional antibodies conserved their Fc domain to be able to bind to cells expressing Fc receptors. (B) BiTEs (bispecific T-cell engagers). (C) BiKEs (bispecific NK-cell engagers). (D) TriKEs (trispecific NK-cell engagers). (E) Single-domain antibodies only have one variable chain per target, they are usually made from heavy chain nanobodies derived from the structure of heavy-chain only camelid antibodies. (F) HLE BiTEs (half-life extended bispecific T-cell engagers) are BiTEs with an Fc portion that increases its half-life. (G) DARTs (dual affinity retargeting antibodies). Created with BioRender.com.
The first application of bsAbs in cancer immunotherapy was focused on leading T cells toward tumor cells by the interaction between the extracellular subunit of CD3 on T cells and cancer-related antigens. The bsAbs ease the interaction of the major histocompatibility complex (MHC) with its cognate T-cell receptor (TCR) resulting in a proper T-cell priming and activation. Despite this, some adverse effects of these drugs such as cytokine release syndrome or liver toxicity and other limitations such as a short half-life have been reported. For that matter a vast quantity of clinical trials with these molecules is being conducted [4].
Nevertheless, bsAbs still represent a novel and effective approach to treat cancer patients because they target molecules expressed on the surface of cancer cells (tumor-associated antigens [TAAs]) and bind to specific receptors that are located on effector cells of the immune system (Figure 2) [5][6]. Furthermore, there have been other smart approaches for the use of bsAbs. Fournier et al. used the Newcastle Disease Virus to specifically infect cancer cells and make them express viral antigens such as hemagglutinin-neuraminidase and fusion molecules. By expressing these viral antigens, bsAbs can be engineered to engage immune effector cells to cancer cells, decreasing the risk of on-target/off-tumor toxicity seen by targeting TAAs that are also expressed in healthy cells such as EGFR or VEGFR [7].
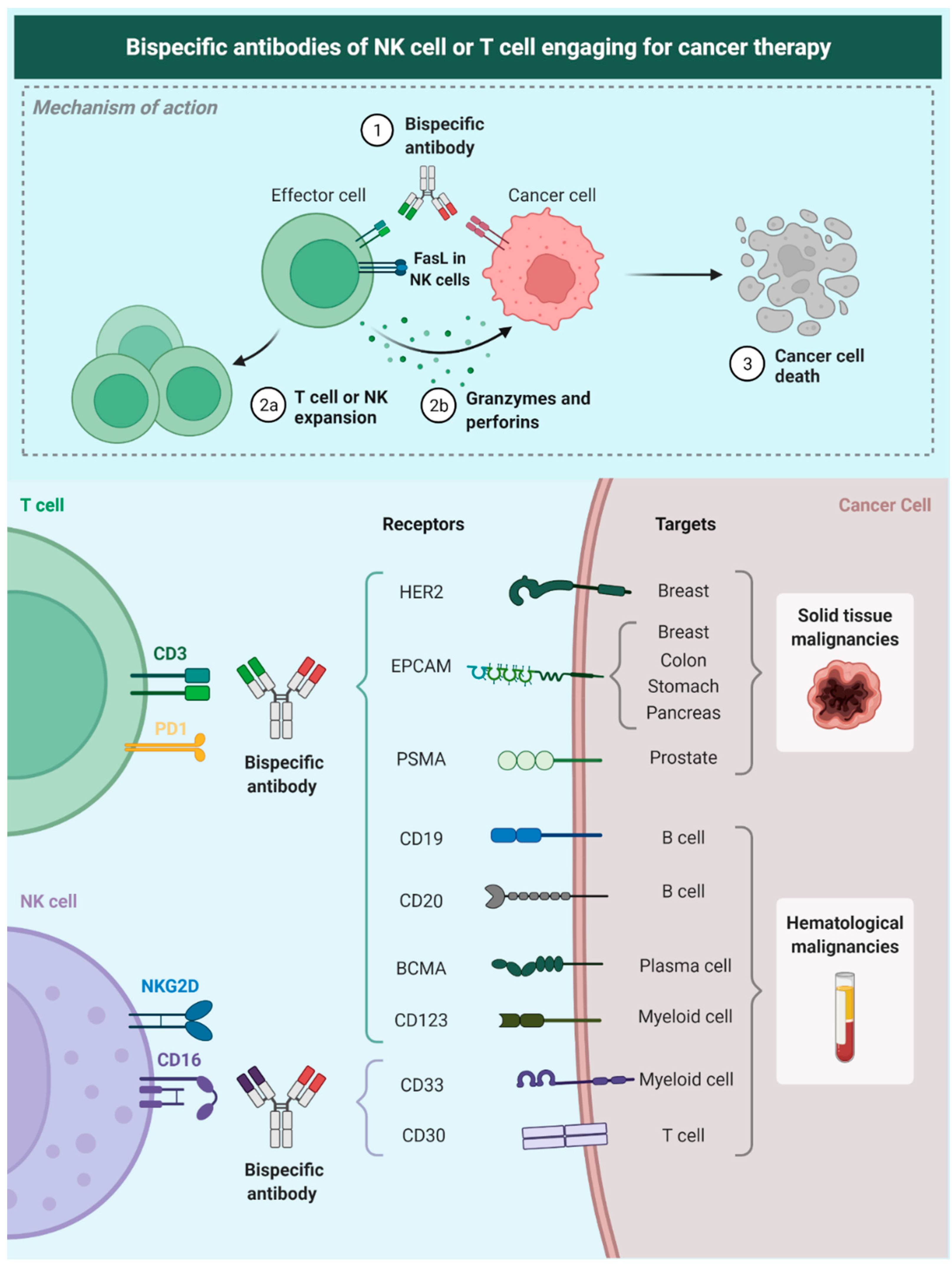
Figure 2. Description of the mechanism of NK-Bias; these antibodies target a tumor-related antigen and bind membrane receptors on NK cells allowing a spatial and molecular immune-mediated cell-killing process. Researchers also show some of the tumor-associated targets that currently have been studied for therapy. Epithelial cell adhesion molecule (EpCAM), epidermal growth factor receptor 2 (HER2), prostate specific membrane antigen (PSMA), B-cell maturation antigen (BCMA), CD19, CD20, CD123, CD33, CD30 (cluster of differentiation [CD]).
Currently, an important number of bsAbs are being studied in many clinical trials, showing positive results in a specific group of tumoral cells and a prolonged antitumoral response. Particularly, some malignancies such as lymphomas seem to have a better antitumoral response with bsAbs, in comparison with myeloid neoplasias or solid tumors [8]. For solid tumors, an optimal antibody impregnation to the tumor has been reported; however, a short half-life and concerns about their safety are still subjects of study [9].
2. Pharmacology of Bispecific Antibodies
Antibodies are molecules made of different structural and functional parts. These parts are combined to create molecules with unique affinity, specificity, and interaction properties. The special structure-function of these macromolecules is beyond the scope of this out of scope; however, understanding some basic principles is essential to comprehend the pharmacology behind bsAbs [10].
From a structural point of view, a bsAbs is made of two identical light chains (LCs) and two identical heavy chains (HCs). Each domain, between the LC and HC has disulfide bonds. The structural conformation creates three zones: two antigen-binding fragments (Fab) and one crystallizable fragment or Fc. Both Fab regions bind to molecular targets, the same epitope. On the other hand, the Fc region attaches to receptors such as Fcγ receptors (FcγRs), C1q, and neonatal Fc receptor (FcRn), mediating its effector functions [10][11].
Previously, it was mentioned that although bsAbs contain two Fab regions, these only bind to the same epitope in an antigen, defining monospecificity. As the name suggests, bsAbs are bispecific, because they bind to two antigens. Structurally, there are two principal types of bsAbs: (1) single-chain variable fragment (scFv) antibodies and (2) full-length IgG-based antibody. In the past time, three techniques were used for their creation bispecific T-cell engager (BiTE), dual-affinity retargeting proteins (DARTs) and tandem diabodies (TandAbs) [12]. Currently, they are created by orthogonal Fab interface, DuoBody, XmAb, CrossMab, and knobs-into-holes (KiH) [3].
Mechanisms of action are diverse. First, the process of binding immune cells with tumor cells, leads to suppression of malignant cells’ ability to escape the immune response. Second, bsAbs decrease the expression of certain molecules and the release of immune suppression mediators. Additionally, bsAbs also block targets such as PD1, CTLA-4, LAG-3, IL-23, TNF-a, and others and they also stimulate immune cells, those mechanisms act synergistically [13][14].
From a pharmacokinetic (PK) view the properties of bsAbs oscillate due to their different compositions. The Fc region plays a key role in bsAbs PK. It is reported that bioavailability is lower by oral administration, so other ways of administration are better choices.
With respect of the distribution, three parameters affect this variable: extravasation, distribution within a tissue, and elimination/clearance. Extravasation occurs when macromolecules have a high volume of distribution (Vd) and tend to remain in the tissues. Additionally, it is known that intracellular catabolism and renal clearance, both influenced by Fc region, increase the molecular weight of some molecules leading to slow clearance. After that, those molecules bind to FcRn, escape acidic endosomes, and return to the circulation or to the interstitial space. In the case of specific elimination, bsAbs bind to specific antigens on cell surfaces mediating its clearance in a process named target-mediated drug disposition (TMDD). In an attempt to integrate some of the previous concepts, scFvs vs. full-length IgG-based bsAbs have different molecular weights; this distinction determines the route of clearance and the time in circulation and route of administration [15][16].
BsAbs pharmacology is complicated, many situations and variables affect its pharmacodynamics and pharmacokinetics. Understanding the design, development, and properties of these molecules is fundamental for the development of new molecules [14].
3. T-Cell Engaging Bispecific Antibodies
Among all the antibody-centered cancer therapy, T-cell engaging bispecific antibodies (T-biAbs) have a promising role in future cancer therapeutics. These antibodies use the main principle of two different binding arms of the bispecific antibodies. One of the binding sites recognizes the invariable CD3 subunits of cytotoxic T lymphocytes (CTLs) and the other one recognizes certain tumor antigens. Therefore, the T-biAb can activate CTLs bypassing the MHC pathway and redirecting this activation to attack specific cancer cells [17]. Currently, there are two FDA-approved T-biAbs blinatumomab (for the management of acute lymphoblastic leukemia) [18][19].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14061243
References
- Nuñez-Prado, N.; Compte, M.; Harwood, S.; Álvarez-Méndez, A.; Lykkemark, S.; Sanz, L.; Álvarez-Vallina, L. The Coming of Age of Engineered Multivalent Antibodies. Drug Discov. Today 2015, 20, 588–594.
- Labrijn, A.; Janmaat, M.; Reichert, J.; Parren, P. Bispecific Antibodies: A Mechanistic Review of the Pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608.
- Viardot, A.; Bargou, R. Bispecific Antibodies in Haematological Malignancies. Cancer Treat. Rev. 2018, 65, 87–95.
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat. Rev. Immunol. 2020, 20, 651–668.
- Trabolsi, A.; Arumov, A.; Schatz, J.H.; Alerts, E. T Cell—Activating Bispecific Antibodies in Cancer Therapy. J. Immunol. 2022, 203, 585–592.
- Blanco, B.; Compte, M.; Lykkemark, S.; Sanz, L.; Alvarez-Vallina, L. T Cell-Redirecting Strategies to ‘sTAb’ Tumors: Beyond CARs and Bispecific Antibodies. Trends Immunol. 2019, 40, 243–257.
- Fournier, P.; Schirrmacher, V. Bispecific Antibodies and Trispecific Immunocytokines for Targeting the Immune System Against Cancer. BioDrugs 2013, 27, 35–53.
- Duell, J.; Lammers, P.E.; Djuretic, I.; Chunyk, A.G.; Alekar, S.; Jacobs, I.; Gill, S. Bispecific Antibodies in the Treatment of Hematologic Malignancies. Clin. Pharmacol. Ther. 2019, 106, 781–791.
- Wu, Y.; Yi, M.; Zhu, S.; Wang, H.; Wu, K. Recent Advances and Challenges of Bispecific Antibodies in Solid Tumors. Exp. Hematol. Oncol. 2021, 10, 56.
- Goulet, D.R.; Atkins, W.M. Considerations for the Design of Antibody-Based Therapeutics. J. Pharm. Sci. 2021, 109, 74–103.
- Chiu, M.; Goulet, D.; Teplyakov, A.; Gilliland, G. A Review of Bispecific Antibodies and Antibody Constructs in Oncology and Clinical Challenges. Pharmacol. Ther. 2019, 201, 103–119.
- Wang, Q.; Chen, Y.; Park, J.; Liu, X.; Hu, Y.; Wang, T.; Mcfarland, K.; Betenbaugh, M.J. Design and Production of Bispecific Antibodies.Pdf. Antibodies 2019, 8, 43.
- Wang, S.; Chen, K.; Lei, Q.; Ma, P.; Yuan, A.Q.; Zhao, Y.; Jiang, Y.; Fang, H.; Xing, S.; Fang, Y.; et al. The State of the Art of Bispecific Antibodies for Treating Human Malignancies. EMBO Mol. Med. 2021, 13, e14291.
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific Antibodies: From Research to Clinical Application. Front. Immunol. 2021, 12, 626616.
- Lobo, E.D.; Hansen, R.J.; Balthasar, J.P. Antibody Pharmacokinetics and Pharmacodynamics. J. Pharm. Sci. 2004, 93, 2645–2668.
- Chen, Y. Pharmacokinetics of Bispecific Antibody. Curr. Pharmacol. Rep. 2017, 3, 126–137.
- Wu, Z.; Cheung, N. T Cell Engaging Bispecific Antibody (T-BsAb): From Technology to Therapeutics. Pharmacol. Ther. 2018, 182, 161–175.
- Linke, R.; Klein, A.; Seimetz, D. Clinical Development and Future Directions Catumaxomab. Mabs 2010, 2, 129–136.
- Chow, V. Clinical Use of Blinatumomab for B-Cell Acute Lymphoblastic Leukemia in Adults. Ther. Clin. Risk Manag. 2016, 12, 1301–1310.
This entry is offline, you can click here to edit this entry!
