Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Xanthones are considered polyketide derivatives due to their biosynthetic precursor. They are aromatic oxygenated heterocyclic compounds with a dibenzo-γ-pyrone scaffold, known as 9H-xanthen-9-one.
- xanthones
- marine-derived xanthones
- bioactive xanthones
1. Introduction
Xanthones are considered polyketide derivatives due to their biosynthetic precursor. They are aromatic oxygenated heterocyclic compounds with a dibenzo-γ-pyrone scaffold, known as 9H-xanthen-9-one (Figure 1) [1]. This molecule can accommodate various substituents at different positions [2], making xanthones recognized as privileged scaffolds in searching for new drugs [1][3].
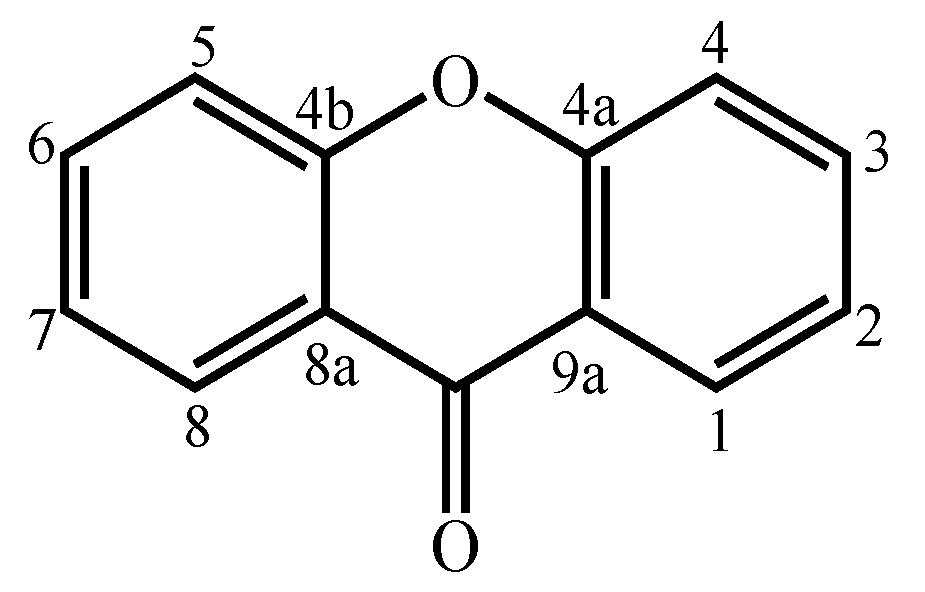 Figure 1. Xanthone (9H-xanthen-9-one) core structure.
Figure 1. Xanthone (9H-xanthen-9-one) core structure.Xanthones are widely distributed in nature in higher plants, lichens, and fungi from terrestrial origins [4][5][6][7][8][9]. In addition, the marine environment, the least explored area of this planet, has also proven to be an invaluable source [10][11]. Many xanthones have been isolated from marine-derived fungi [10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40], which can be found in marine sediments and associated with other marine organisms [12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40]. Marine environments, such as temperature, salinity, and pressure, to which marine organisms are subject, sometimes force them to develop unique defenses against the conditions in which they live [41][42]. These unique defenses can lead to the biosynthesis of new secondary metabolites, different from those synthesized by terrestrial sources [41]. The chemically different/unique structures allow xanthones to have important biological activities, such as cytotoxic [12][15][16][17][21][22][23][27][32][37][43], antibacterial [12][16][17][18][19][20][22][26][29][30][35][36][39], and antifungal [20][22][33][40] activities, giving them great potential as natural products with medicinal value [1].
Therefore, the chemical uniqueness of marine-derived xanthones and the significant bioactivities support the idea that the marine environment could be a valuable source of new hits, leads, and drugs [44]. However, access to some marine natural products may be difficult, and in some cases, isolation may yield small amounts of compounds [45]. Different techniques have been applied to increase the availability of the new natural compounds, which can be produced by bacteria or yeasts, or through a chemical approach, such as laboratory synthesis [45]. Synthetic routes can help to overcome supply problems, but it is also very important since it allows the preparation of structures with different substitution patterns relative to those provided by nature, which rise to the opportunity to generate new bioactive agents [46][47][48]. Bioinspiration is very useful in medicinal chemistry as it allows the selection of molecular structures to be used as scaffolds and the strategy to follow for molecular transformations [2].
2. New Marine Xanthones Isolated since 2010
Under the period covered by this research, several studies were carried out to identify biologically active marine compounds, of which the xanthones stand out. Around 100 xanthones were isolated, from which 51 are considered new natural compounds.
Nowadays, the isolation of xanthone derivatives from marine sources usually involves fermentation to increase the compound’s final amount. Fermentation involves putting the fungi in a culture broth, which comprises all the nutrients, such as glucose, iron phosphate, calcium carbonate, malt extract, and controlled pH [21][26], necessary for its growth. The duration of fermentation can be variable but usually involve several days, at least 14 days and the highest 45 days [14][17]. Then, the cultured broth is filtered and extracted, most often, with EtOAc. This extract is subjected to a vacuum liquid chromatography over a silica gel column [15][16][21][23], flash chromatography [19] or, most commonly, to normal column chromatography (CC) [14][17][18][20][22], originating different fractions, which can be subjected to analysis by HPLC or TLC, obtaining sub-fractions, or be directly purified to obtain pure compounds. Most of the xanthones herein referred to were obtained after semi-preparative HPLC purifications of these sub-fractions. Naturally, the eluents and gradients used differ depending on the extracts/fractions being analyzed. The most common eluents used are organic solvents mixtures, such as n-hexane/CH2Cl2/MeOH [16], n-hexane/EtOAc [15], CH2Cl2/MeOH [15][18][20][21][23], gradient of petroleum ether/EtOAc [14][17][18], but mixtures of H2O/MeOH can also be used successfully [19].
The new xanthone derivatives isolated include simple ones (Figure 2), mainly isolated from marine-derived fungi found in marine sediments [15][18][20][31][32][33][34][35][36][46][47][48][49]. These xanthones are usually highly substituted, with different substituent patterns being the substituents found to be essential for four types; hydroxy, methoxy, methyl, and methoxycarbonyl groups. It seems that the methoxy group is not common in bioactive marine xanthones [50], but yicathins B (3) and C (4) (Figure 2), which were recently prepared by total synthesis [51], present such a group at C-8. Other examples include xanthones bearing a 6-methoxy group (2) and (6) and 7-methoxy group (13) and (15) (Figure 2). On the other hand, marine xanthones (14) and (16) present two methoxy groups, respectively, at C-3 and C-7 and at C-2 and C-7 (Figure 2).
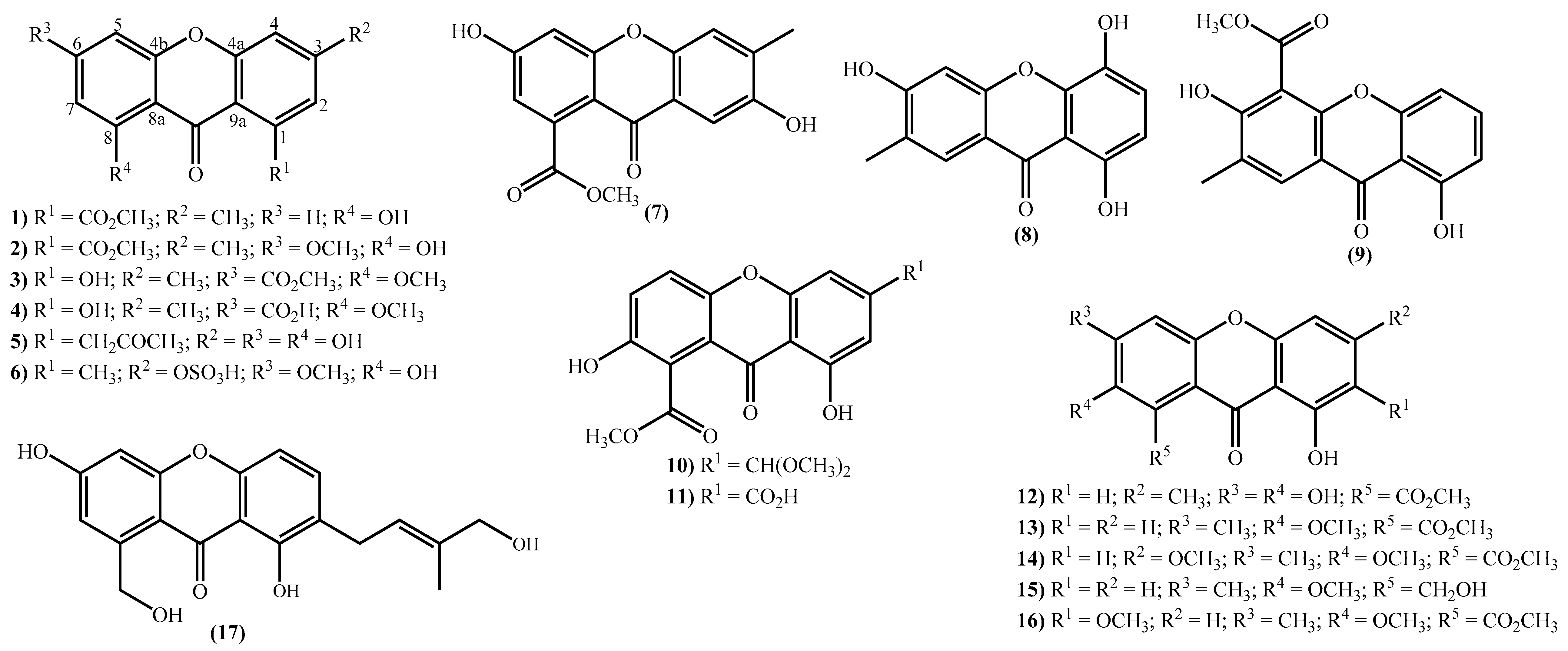 Figure 2. Marine xanthones with simple structures isolated for the first time from 2010 to 2021.
Figure 2. Marine xanthones with simple structures isolated for the first time from 2010 to 2021.The more complex xanthones isolated during the covered period are depicted in Figure 3 and present the xanthone skeleton fused with other rings. The most interesting examples include (2S,3aS,12cS)-8-hydroxy-2,6-dimethoxy-1,2,3a,12c-tetrahydro-7H-furo [3’,2’:4,5] furo [2,3-c] xanthen-7-one (18), isolated from the marine fungus Aspergillus versicolor and named oxisterigmatocystin D due to its similarity with the known oxisterigmatocystin C [37]. Although the similarities with oxisterigmatocystin C and other isomers were previously isolated, the researchers established the stereogenic centers’ configurations and demonstrated that oxisterigmatocystin D is a new natural compound [37]. Compound (19), named monacyclione G, is another example isolated from Streptomyces sp. HDN15129 [21]. Its stereochemistry was confirmed by the NMR data mainly through the correlations found in HMBC [21] and was established as depicted in Figure 3.
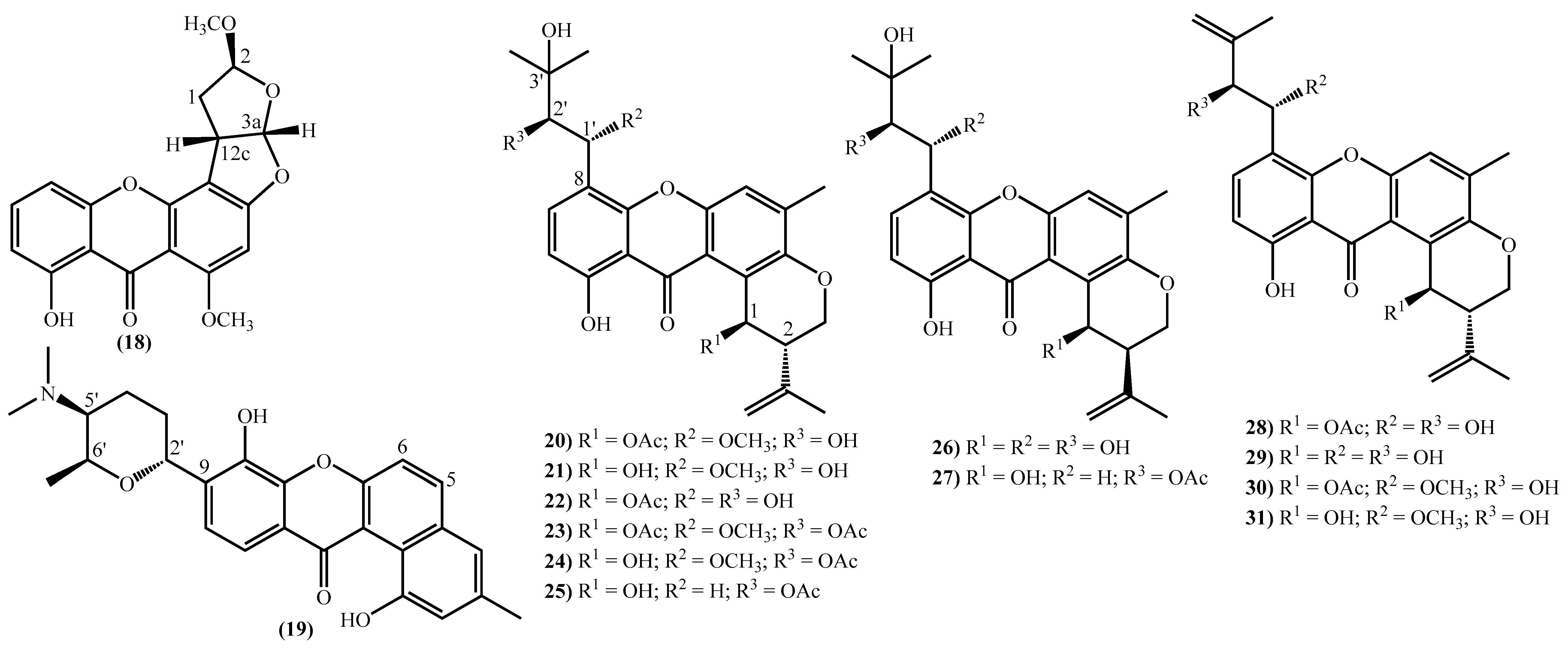
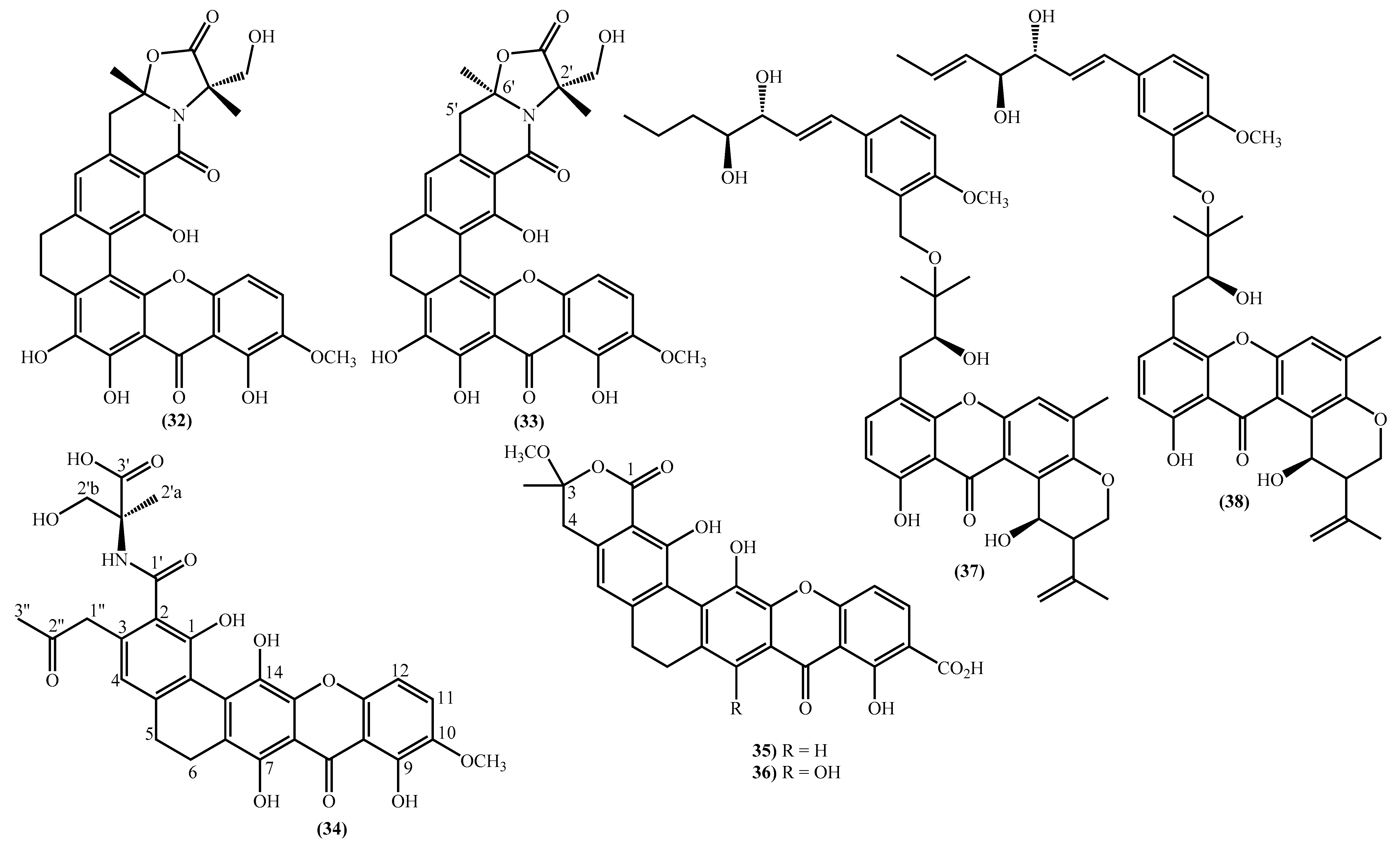 Figure 3. Marine xanthones with more complex structures isolated for the first time from 2010 to 2021.
Figure 3. Marine xanthones with more complex structures isolated for the first time from 2010 to 2021.The most prevalent nucleus in these new marine xanthones is the 11-hydroxy-5-methyl-2-(prop-1-en-2-yl)-2,3-dihydropyrano [3,2-a]xanthen-12(1H)-one, found in fourteen of the twenty-one new derivatives (Figure 3). Twelve marine xanthones with this core were isolated from the fungus Aspergillus sp. ZA-01, respectively, aspergixanthones A-K (20–24 and 26–31) and the 15-acetyltajixanthone hydrate (25) (Figure 3) [14][17]. Varioxiranols F (37) and G (38) (Figure 3) were isolated from the Emericella variecolor, a fungus associated with a Cinachyrella sp. Sponge [28].
The aspergixanthones (20–24, 26 and 27) and the 15-acetyltajixanthone hydrate (25) have a 3-hydroxy-3-methylbutyl moiety at C-8, whereas the remaining aspergixanthones (28–31) have at the same position a 3-methylbut-3-en-1-yl substituent (Figure 3), in both cases, hydroxy, methoxy or acetyloxy groups at C-1′ and C-2′ are also present (Figure 3).
The last examples are xanthone derivatives bearing a 1,7,8,10-tetrahydroxy-11-methoxy-5,6-dihydro-9H-naphtho [2,1-c]xanthen-9-one (32, 33) and a 1,9,14-trihydroxy-5,6-dihydro-8H-naphtho [2,1-b]xanthen-8-one nucleus (34–36) (Figure 3). Buanmycin (34) was extracted from a marine Streptomyces cyaneus [52], and the remaining examples, citreamicin θ A (32), citreamicin θ B (33), citreaglycon A (36), and dehydrocitreaglycon A (35), were isolated from Streptomyces caelestis [19].
In the last years, complex tetrahydroxanthone derivatives were also isolated (Figure 4) [23][26][27][40]. Compounds (39–44), named versixanthones A-F, were isolated from the fungus Aspergillus versicolor HDN1009 [27]. Compounds (45, 46) were isolated from the fungus Engyodontium album strain LF069 [26] and the Tritirachium sp. SpB081112MEf2 a marine sponge fungus [40]. Finally, compounds (47–51) were also isolated from the fungus Aspergillus versicolor HDN1009 [23].
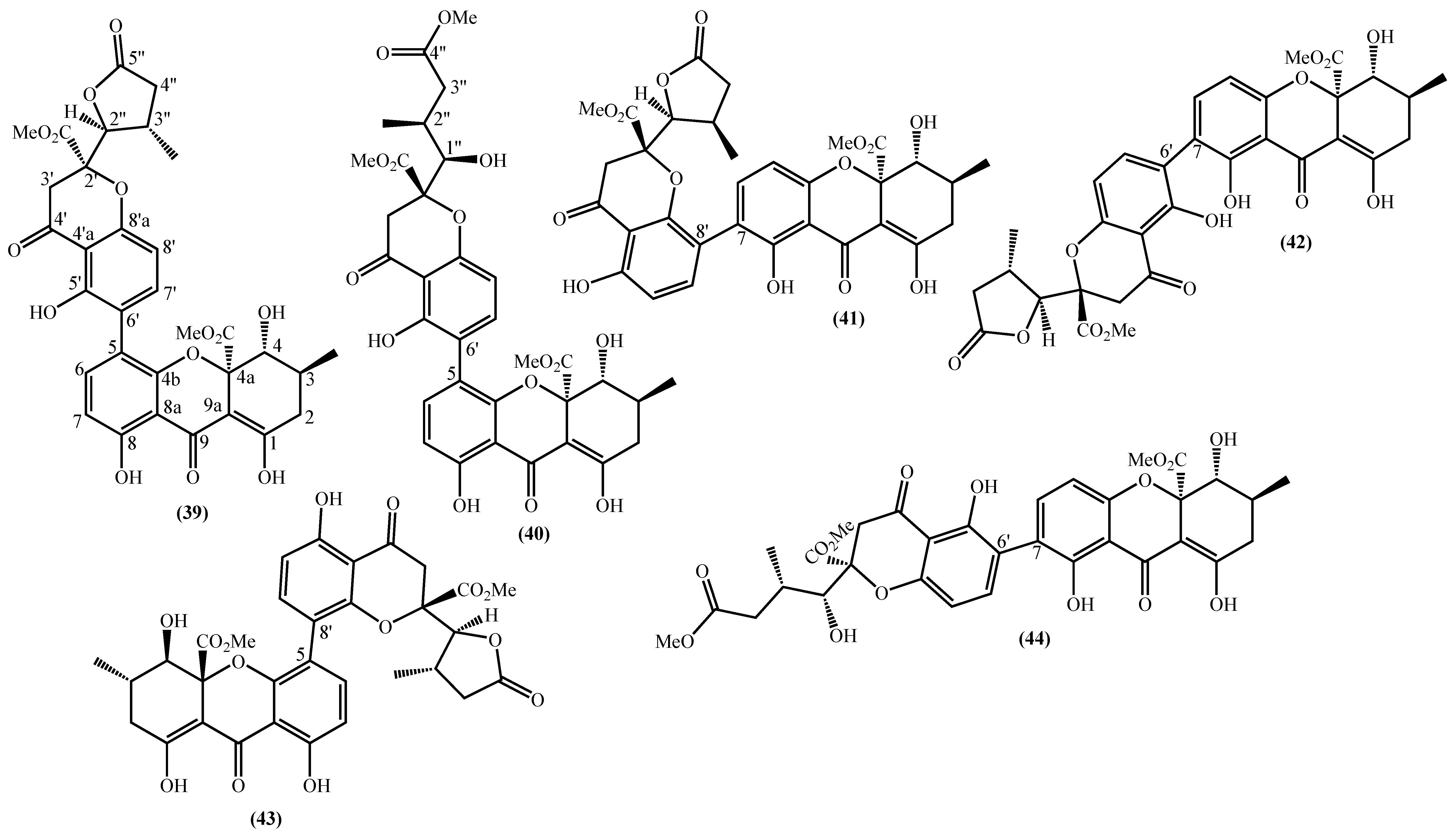
 Figure 4. Marine tetrahydroxanthones isolated for the first time from 2010 to 2021.
Figure 4. Marine tetrahydroxanthones isolated for the first time from 2010 to 2021.Versixanthones A–F have a methyl (3R,4S,4aS)-1,4,8-trihydroxy-3-methyl-9-oxo-2,3,4,9-tetrahydro-4aH-xanthene-4a-carboxylate moiety linked to a methyl (2′R,2″S,3″S)-5-hydroxy-2-(3-methyl-5-oxotetrahydrofuran-2-yl)-4-chromanone-2-carboxylate (39, 41–43) and methyl (2′R,1″R,2″S)-5-hydroxy-2-(1-hydroxy-4-methoxy-2-methyl-4-oxobutyl)-4-chromanone-2-carboxylate (40, 44). The main differences are the linkage between the two moieties since the researchers establish the stereocenter configurations as equal in all isomers (Figure 4) [27].
Compounds (45, 46) represent two diastereomers fully characterized by Wu et al. in 2016 [26], although the isomers JBIR-99 (45a) and JBIR-97/98 (46a) were previously isolated by Ueda et al. in 2010 [40]. The absolute configuration of these isomers and the engyodontochone A (46b) and B (45b) was established using NMR experiments and is depicted in Figure 4 [26].
The final examples are the dimeric tetrahydroxanthones (47–51) isolated from the fungus Aspergillus versicolor HDN1009 and named versixanthones G-K (Figure 4) [23]. The structure depicted in Figure 4, including the absolute configuration, was established using NMR experiments.
This entry is adapted from the peer-reviewed paper 10.3390/md20060347
References
- Khattab, A.R.; Farag, M.A. Marine and terrestrial endophytic fungi: A mine of bioactive xanthone compounds, recent progress, limitations, and novel applications. Crit. Rev. Biotechnol. 2022, 42, 403–430.
- Pinto, M.M.M.; Palmeira, A.; Fernandes, C.; Resende, D.I.S.P.; Sousa, E.; Cidade, H.; Tiritan, M.E.; Correia-da-Silva, M.; Cravo, S. From natural products to new synthetic small molecules: A journey through the world of xanthones. Molecules 2021, 26, 431.
- Panda, S.S.; Chand, M.; Sakhuja, R.; Jain, S.C. Xanthones as potential antioxidants. Curr. Med. Chem. 2013, 20, 4481–4507.
- Negi, J.S.; Bisht, V.K.; Singh, P.; Rawat, M.S.M.; Joshi, G.P. Naturally occurring xanthones: Chemistry and biology. J. Appl. Chem. 2013, 2013, 621459.
- Masters, K.-S.; Bräse, S. Xanthones from fungi, lichens, and bacteria: The natural products and their synthesis. Chem. Rev. 2012, 112, 3717–3776.
- Resende, D.; Pereira-Terra, P.; Inácio, Â.; Costa, P.; Pinto, E.; Sousa, E.; Pinto, M. Lichen xanthones as models for new antifungal agents. Molecules 2018, 23, 2617.
- Peres, V.; Nagem, T.J.; de Oliveira, F.F. Tetraoxygenated naturally occurring xanthones. Phytochemistry 2000, 55, 683–710.
- Khattab, A.R.; Farag, M.A. Current status and perspectives of xanthones production using cultured plant biocatalyst models aided by in-silico tools for its optimization. Crit. Rev. Biotechnol. 2020, 40, 415–431.
- El-Seedi, H.R.; El-Ghorab, D.M.H.; El-Barbary, M.A.; Zayed, M.F.; Göransson, U.; Larsson, S.; Verpoorte, R. Naturally occurring xanthones; latest investigations: Isolation, structure elucidation and chemosystematic significance. Curr. Med. Chem. 2009, 16, 2581–2626.
- Loureiro, D.R.P.; Soares, J.X.; Costa, J.C.; Magalhães, Á.F.; Azevedo, C.M.G.; Pinto, M.M.M.; Afonso, C.M.M. Structures, activities and drug-likeness of anti-Infective xanthone derivatives isolated from the marine environment: A review. Molecules 2019, 24, 243.
- Pinto, M.M.M.; Castanheiro, R.A.P.; Kijjoa, A. Xanthones from marine-derived microorganisms: Isolation, structure elucidation and biological activities. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 1–21.
- Lee, Y.M.; Li, H.; Hong, J.; Cho, H.Y.; Bae, K.S.; Kim, M.A.; Kim, D.-K.; Jung, J.H. Bioactive metabolites from the sponge-derived fungus Aspergillus versicolor. Arch. Pharm. Res. 2010, 33, 231–235.
- Tan, S.; Yang, B.; Liu, J.; Xun, T.; Liu, Y.; Zhou, X. Penicillixanthone A, a marine-derived dual-coreceptor antagonist as anti-HIV-1 agent. Nat. Prod. Res. 2019, 33, 1467–1471.
- Zhu, A.; Zhang, X.-W.; Zhang, M.; Li, W.; Ma, Z.-Y.; Zhu, H.-J.; Cao, F. Aspergixanthones I–K, new anti-vibrio prenylxanthones from the marine-derived fungus Aspergillus sp. ZA-01. Mar. Drugs 2018, 16, 312.
- Elnaggar, M.S.; Ebada, S.S.; Ashour, M.L.; Ebrahim, W.; Müller, W.E.G.; Mándi, A.; Kurtán, T.; Singab, A.; Lin, W.; Liu, Z.; et al. Xanthones and sesquiterpene derivatives from a marine-derived fungus Scopulariopsis sp. Tetrahedron 2016, 72, 2411–2419.
- Li, J.L.; Jiang, X.; Liu, X.; He, C.; Di, Y.; Lu, S.; Huang, H.; Lin, B.; Wang, D.; Fan, B. Antibacterial anthraquinone dimers from marine derived fungus Aspergillus sp. Fitoterapia 2019, 133, 1–4.
- Zhu, A.; Yang, M.-Y.; Zhang, Y.-H.; Shao, C.-L.; Wang, C.-Y.; Hu, L.-D.; Cao, F.; Zhu, H.-J. Absolute configurations of 14,15-hydroxylated prenylxanthones from a marine-derived Aspergillus sp. fungus by chiroptical methods. Sci. Rep. 2018, 8, 10621.
- Lv, H.; Wang, K.; Xue, Y.; Chen, J.; Su, H.; Zhang, J.; Wu, Y.; Jia, J.; Bi, H.; Wang, H.; et al. Three new metabolites from the marine-derived fungus Aspergillus sp. WHUF03110. Nat. Prod. Commun. 2021, 16, 1934578X2110550.
- Liu, L.-L.; Xu, Y.; Han, Z.; Li, Y.-X.; Lu, L.; Lai, P.-Y.; Zhong, J.-L.; Guo, X.-R.; Zhang, X.-X.; Qian, P.-Y. Four new antibacterial xanthones from the marine-derived Actinomycetes Streptomyces caelestis. Mar. Drugs 2012, 10, 2571–2583.
- Ningsih, B.N.S.; Rukachaisirikul, V.; Pansrinun, S.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. New aromatic polyketides from the marine-derived fungus Pseudopithomyces maydicus PSU-AMF350 and their antimicrobial activity. Nat. Prod. Res. 2022, 36, 1–8.
- Chang, Y.; Xing, L.; Sun, C.; Liang, S.; Liu, T.; Zhang, X.; Zhu, T.; Pfeifer, B.A.; Che, Q.; Zhang, G.; et al. Monacycliones G–K and ent-gephyromycin A, angucycline derivatives from the marine-derived Streptomyces sp. HDN15129. J. Nat. Prod. 2020, 83, 2749–2755.
- Fredimoses, M.; Zhou, X.; Ai, W.; Tian, X.; Yang, B.; Lin, X.; Liu, J.; Liu, Y. Emerixanthone E, a new xanthone derivative from deep sea fungus Emericella sp SCSIO 05240. Nat. Prod. Res. 2019, 33, 2088–2094.
- Wu, G.; Qi, X.; Mo, X.; Yu, G.; Wang, Q.; Zhu, T.; Gu, Q.; Liu, M.; Li, J.; Li, D. Structure-based discovery of cytotoxic dimeric tetrahydroxanthones as potential topoisomerase I inhibitors from a marine-derived fungus. Eur. J. Med. Chem. 2018, 148, 268–278.
- Luo, X.; Yang, J.; Chen, F.; Lin, X.; Chen, C.; Zhou, X.; Liu, S.; Liu, Y. Structurally diverse polyketides from the mangrove-derived fungus Diaporthe sp. SCSIO 41011 with their anti-influenza A virus activities. Front. Chem. 2018, 6, 282.
- Liu, H.; Chen, S.; Liu, W.; Liu, Y.; Huang, X.; She, Z. Polyketides with immunosuppressive activities from mangrove endophytic fungus Penicillium sp. ZJ-SY2 Hongju. Mar. Drugs 2016, 14, 217.
- Wu, B.; Wiese, J.; Wenzel-Storjohann, A.; Malien, S.; Schmaljohann, R.; Imhoff, J.F. Engyodontochones, antibiotic polyketides from the marine fungus Engyodontium album strain LF069. Chem.-Eur. J. 2016, 22, 7452–7462.
- Wu, G.; Yu, G.; Kurtán, T.; Mándi, A.; Peng, J.; Mo, X.; Liu, M.; Li, H.; Sun, X.; Li, J.; et al. Versixanthones A–F, cytotoxic xanthone–chromanone dimers from the marine-derived fungus Aspergillus versicolor HDN1009. J. Nat. Prod. 2015, 78, 2691–2698.
- Wu, Q.; Wu, C.; Long, H.; Chen, R.; Liu, D.; Proksch, P.; Guo, P.; Lin, W. Varioxiranols A–G and 19-O-methyl-22-methoxypre-shamixanthone, PKS and hybrid PKS-derived metabolites from a sponge-associated Emericella variecolor fungus. J. Nat. Prod. 2015, 78, 2461–2470.
- Jang, N.Y.; Peng, F.; Fang, Z.K.; Wu, Y.D.; Chen, M.H.; Xie, Y.; Wang, H.R.; Jiang, H.; Lian, Y.Y. Study on aromatic polyketide metabolite with antibacterial activity from the marine-derived Actinomadura sp. FIM95-F26. Chin. J. Antibiot. 2015, 40, 161–165.
- Eltamany, E.E.; Abdelmohsen, U.R.; Ibrahim, A.K.; Hassanean, H.A.; Hentschel, U.; Ahmed, S.A. New antibacterial xanthone from the marine sponge-derived Micrococcus sp. EG45. Bioorg. Med. Chem. Lett. 2014, 24, 4939–4942.
- Sun, Y.-L.; Zhang, X.-Y.; Zheng, Z.-H.; Xu, X.-Y.; Qi, S.-H. Three new polyketides from marine-derived fungus Penicillium citrinum SCSGAF 0167. Nat. Prod. Res. 2014, 28, 239–244.
- Yang, J.X.; Qiu, S.; She, Z.; Lin, Y. A new xanthone derivative from the marine fungus Phomopsis sp. (No. SK7RN3G1). Chem. Nat. Compd. 2013, 49, 246–248.
- Li, C.; Zhang, J.; Shao, C.; Ding, W.; She, Z.; Lin, Y. A new xanthone derivative from the co-culture broth of two marine fungi (strain No. E33 and K38). Chem. Nat. Compd. 2011, 47, 382–384.
- Gao, H.; Zhou, L.; Li, D.; Gu, Q.; Zhu, T.-J. New cytotoxic metabolites from the marine-derived fungus Penicillium sp. ZLN29. Helv. Chim. Acta 2013, 96, 514–519.
- Li, H.-L.; Li, X.-M.; Liu, H.; Meng, L.-H.; Wang, B.-G. Two New diphenylketones and a new xanthone from Talaromyces islandicus EN-501, an endophytic fungus derived from the marine red alga Laurencia okamurai. Mar. Drugs 2016, 14, 223.
- Song, Z.; Gao, J.; Hu, J.; He, H.; Huang, P.; Zhang, L.; Song, F. One new xanthenone from the marine-derived fungus Aspergillus versicolor MF160003. Nat. Prod. Res. 2020, 34, 2907–2912.
- Wu, Z.-H.; Liu, D.; Xu, Y.; Chen, J.-L.; Lin, W.-H. Antioxidant xanthones and anthraquinones isolated from a marine-derived fungus Aspergillus versicolor. Chin. J. Nat. Med. 2018, 16, 219–224.
- Liu, S.; Wang, H.; Su, M.; Hwang, G.J.; Hong, J.; Jung, J.H. New metabolites from the sponge-derived fungus Aspergillus sydowii J05B-7F-4. Nat. Prod. Res. 2017, 31, 1682–1686.
- Wang, Y.; Lin, X.-P.; Ju, Z.-R.; Liao, X.-J.; Huang, X.-J.; Zhang, C.; Zhao, B.-X.; Xu, S.-H. Aspergchromones A and B, two new polyketides from the marine sponge-associated fungus Aspergillus sp. SCSIO XWS03F03. J. Asian Nat. Prod. Res. 2017, 19, 684–690.
- Ueda, J.; Takagi, M.; Shin-ya, K. New xanthoquinodin-like compounds, JBIR-97, -98 and -99, obtained from marine sponge-derived fungus Tritirachium sp. SpB081112MEf2. J. Antibiot. 2010, 63, 615–618.
- Jiménez, C. Marine natural products in medicinal chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961.
- Wilson, Z.E.; Brimble, M.A. Molecules derived from the extremes of life. Nat. Prod. Rep. 2009, 26, 44–71.
- Liu, B.; Wang, H.-F.; Zhang, L.-H.; Liu, F.; He, F.-J.; Bai, J.; Hua, H.-M.; Chen, G.; Pei, Y.-H. New compound with DNA Topo I inhibitory activity purified from Penicillium oxalicum HSY05. Nat. Prod. Res. 2015, 29, 2197–2202.
- Shinde, P.; Banerjee, P.; Mandhare, A. Marine natural products as source of new drugs: A patent review (2015–2018). Expert Opin. Ther. Pat. 2019, 29, 283–309.
- Harvey, A. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901.
- Khamthong, N.; Rukachaisirikul, V.; Tadpetch, K.; Kaewpet, M.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Tetrahydroanthraquinone and xanthone derivatives from the marine-derived fungus Trichoderma aureoviride PSU-F95. Arch. Pharm. Res. 2012, 35, 461–468.
- Liu, T.; Zhang, L.; Li, Z.; Wang, Y.; Tian, L.; Pei, Y.; Hua, H. A new sulfo-xanthone from the marine-derived fungus Penicillium sacculum. Chem. Nat. Compd. 2012, 48, 771–773.
- Sun, R.-R.; Miao, F.-P.; Zhang, J.; Wang, G.; Yin, X.-L.; Ji, N.-Y. Three new xanthone derivatives from an algicolous isolate of Aspergillus wentii. Magn. Reson. Chem. 2013, 51, 65–68.
- Liu, F.; Lin, X.; Zhou, X.; Chen, M.; Huang, X.; Yang, B.; Tao, H. Xanthones and quinolones derivatives produced by the deep-sea-derived fungus Penicillium sp. SCSIO Ind16F01. Molecules 2017, 22, 1999.
- Soares, J.X.; Loureiro, D.R.P.; Dias, A.L.; Reis, S.; Pinto, M.M.M.; Afonso, C.M.M. Bioactive marine xanthones: A review. Mar. Drugs 2022, 20, 58.
- Loureiro, D.R.P.; Magalhães, Á.F.; Soares, J.X.; Pinto, J.; Azevedo, C.M.G.; Vieira, S.; Henriques, A.; Ferreira, H.; Neves, N.; Bousbaa, H.; et al. Yicathins B and C and analogues: Total synthesis, lipophilicity and biological activities. ChemMedChem 2020, 15, 749–755.
- Moon, K.; Chung, B.; Shin, Y.; Rheingold, A.L.; Moore, C.E.; Park, S.J.; Park, S.; Lee, S.K.; Oh, K.-B.; Shin, J.; et al. Pentacyclic antibiotics from a tidal mud flat-derived actinomycete. J. Nat. Prod. 2015, 78, 524–529.
This entry is offline, you can click here to edit this entry!
