Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Organic
Fragrances, short for fragrance ingredients, is a type of compounds with a sweet smell or pleasant odor that has wide applications in the fine chemical industry, especially in perfumes, cosmetics, detergents and food additives. Since the discovery of the Diels–Alder reaction, the cycloaddition of π reactants serves as one of the most powerful methods for the construction of carbocycles, which has a broad application in the fragrance industry.
- cycloaddition
- carbocycles
- fragrances
- alkenes
- alkynes
1. Introduction
Fragrances, short for fragrance ingredients, is a type of compounds with a sweet smell or pleasant odor that has wide applications in the fine chemical industry, especially in perfumes, cosmetics, detergents and food additives [1,2,3,4]. The perception of aroma and odor is a subjective phenomenon that is more difficult to describe precisely and measure quantitatively than the perception of light or sound [5,6,7,8,9,10,11,12]. Thus, fragrances with different structures are highly desirable for yielding various scents with repeated effects and long shelf life; they are key to studying the principle of olfaction, including the range and resolution of the olfactory system and the working mechanism of odorant receptors [2,5,6,7,8,9,10,11,12,13,14,15,16,17].
In ancient times, fragrances were mainly obtained from natural resources such as plant essential oils and animal secretions [3,4,18,19]. While natural ingredients still represent an important class of fragrances, they cannot meet the rapidly growing and diversified needs of consumers and perfumers. The power of organic synthesis for fragrances was unlocked in 1868 when coumarin was successfully prepared by W. H. Perkin from salicylaldehyde. With the blossoming of organic synthetic methods and the related industrial technologies over the past century, synthetic and semi-synthetic fragrances emerged to become mainstream in the flavors and fragrances industries [2,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Nowadays, fragrance molecules represent an attractive testing ground and offer a multi-billion-dollar outlet for organic synthesis, stimulating researchers in academia and fragrance manufacture to seek out new fragrances and explore more efficient, economical, scalable and environmentally friendly synthetic methods.
Similar to medicinal chemistry, some structures have been established as privileged structures in fragrance chemistry, many of which have one or more carbo- or heterocycles (Figure 1). The ring sizes range from that of cyclopropane in olibanic acid to the macrocycles in muscone and civetone. Diverse types of rings, including carbocyclic and heterocyclic, saturated and unsaturated, aromatic and non-aromatic, monocyclic and polycyclic rings exist in both natural and synthetic fragrances. With highly structural diversity and the resulting tunable odor characters, cyclic fragrances have emerged as molecular probes for exploring olfactory chemical space. New synthetic methods enable much more structural diversity of fragrances, including the scaffold diversity of different rings and their linking modes, as well as the variations of substituents and their replacement by heteroatoms. These compounds include not only derivatives and analogs of natural fragrances but also those compounds with novel scaffolds due to rational design or unexpected findings.
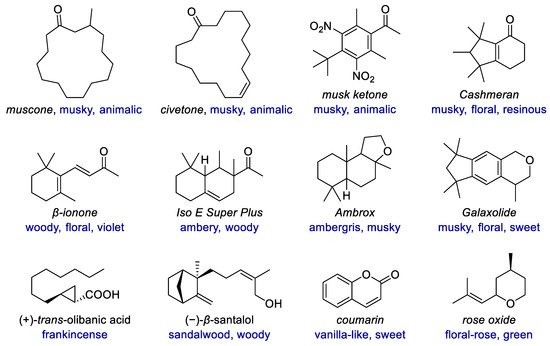
Figure 1. Examples of fragrances with diverse carbocyclic or heterocyclic scaffolds.
Unsaturated hydrocarbons, especially alkenes and alkynes, are extensively used for the construction of carbo- and heterocycles due to their adjustable reactivity and cyclization modes. Many of these building blocks can be easily obtained from natural resources or petrochemicals via reliable processes. In addition, the reactions involving unsaturated bonds usually have high atom economy. With classic or new odorants as targets, innovative synthetic strategies and methods have been developed recently that enrich the frontiers of alkene and alkyne chemistry. Though there are a few reviews published on fragrance synthesis [2,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36], the importance of unsaturated hydrocarbons in this area is still underestimated, especially that of alkynes and enynes.
2. Synthesis of Fragrances via Cycloaddition or Formal Cycloaddition
Since the discovery of the Diels–Alder reaction, the cycloaddition of π reactants serves as one of the most powerful methods for the construction of carbocycles, which has a broad application in the fragrance industry [53]. One classic example is the production of Ambrelux from myrcene via the AlCl3-promoted Diels–Alder reaction [33] (Scheme 1a). Some of the attractive features of cycloaddition include perfect atom economy up to 100%, good functional group compatibility due to typically redox-neutral conditions, and the modular ability to access products via multiple substituents from readily available building blocks.
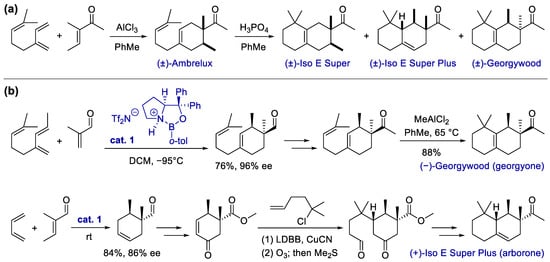
Scheme 1. Synthesis of carbocyclic fragrances via the Diels–Alder reaction. (a) Synthesis of Ambrelux and its conversion via acid-promoted annulative rearrangement; (b) total synthesis of georgyone and arborone, with the enantioselective Diels–Alder reaction as a key step.
Traditional cycloadditions under heating, irradiation or Lewis–acidic conditions are widely used in both laboratory synthesis and industrial fragrance manufacture. In this section, we would like to focus on cyclizations with an organo- or metal-catalyzed process, especially those with unique stereochemical control or cyclization modes. A remarkable example is reported by Hong and Corey, who developed an elegant enantioselective total synthesis of georgyone and arborone [54] (Scheme 1b). The key ring-forming and enantioselective step shared by both routes is a chiral oxazaborolidinium cation-catalyzed Diels–Alder reaction using the corresponding 1,3-butadienes and butenals. The double bond in the adducts facilitates further transformations, including a final annulative isomerization to furnish georgyone and an accelerated 1,4-addition, followed by ozonolysis, to give the keto–aldehyde intermediate toward the production of arborone.
Georgyone and arborone are bicyclic fragrances with intense, clean and transparent woody-ambery note. Interestingly, the name “arborone” (from the Latin word arbor) was first proposed by Corey et al. in their paper, as they found this enantiomer is the key ingredient and deserved an independent term. The racemic form was previously named as “Iso E Super Plus”, which was found unexpectedly as an impurity in Iso E Super that synthesized by an acid-promoted cyclization of Ambrelux [33] (Scheme 1a). Later, another isomeric side product was identified and patented by Givaudan as Georgywood. The odor threshold of (−)-Georgywood was found to be more than 100 times greater than its antipode [55], which stimulated Corey et al. to propose an exclusive term, “georgyone”. Besides these two ingredients, Corey et al. further synthesized several analogs and performed a binding analysis to clarify structural features of woody odorants, including absolute configuration, methyl substitution and spatial orientation. Their work revealed the “magic methyl effect” on this privileged scaffold and opened up an avenue for studying the related olfactory receptors and spatial organization of olfactory glomeruli.
The six-membered ring ester is an attractive scaffold for fragrances with fruity and floral odors, as exemplified by the well-known ethyl safranate. Their odor characteristics have been proven to relate to the double bonds and substituents, but the study of 1,4-cyclohexadiene carboxylates is underdeveloped due to a lack of practical synthetic methods. In 2020, Goeke et al. reported the Co-catalyzed formal [4 + 2] cycloaddition of 1,3-dienes and alkyl propiolates [56] (Scheme 2). The obtained 1,4-cyclohexadiene carboxylates typically demonstrate fruity and green odors, which vary according to their substituents. However, this catalytic system cannot control the regioselectivity of unsymmetrical dienes and unseparated mixture of two regioisomers was obtained.
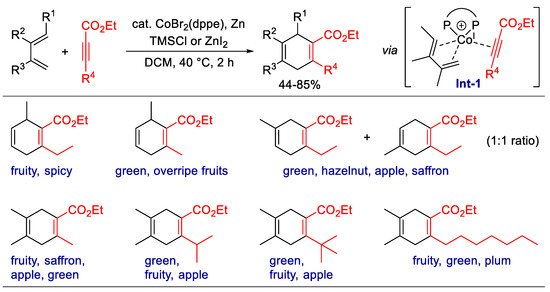
Scheme 2. Synthesis of fragrant 1,4-cyclohexadiene carboxylates via Co-catalyzed [4 + 2] cyclization.
In the proposed catalytic cycle, the in situ formed Co(I) species is coordinated by the diene and alkyne sequentially to form Int-1, which may undergo metallocyclization or follow direct [4 + 2] cycloaddition pathways to form the adduct. The design and screening of ligands would make it possible to achieve regioselectivity or even enantioselectivity. Recently, Shi et al. developed an enantioselective [4 + 2] cycloaddition, catalyzed by a Rh(I) complex with a chiral phosphoramidite ligand [57]. The reaction can be efficiently scaled up by using 26.2 g (E)-1,3-nonadiene and 10 g dimethyl acetylenedicarboxylate to yield the product at 99% ee in 92% yield. However, less active propiolates, such as methyl 2-butynoate, are totally inactive in this reaction, which limits their application for the synthesis of fragrances. Therefore, advanced catalytic systems still need to be developed for the synthesis of fragrant 1,4-cyclohexadienes, with a focus on the improvement of both activity and regioselectivity for unsymmetrically substituted substrates.
Organocatalysis is a powerful toolkit for the construction of C–C bonds and rings via enantioselective addition or cycloaddition. The List group has developed a new type of Brønsted acid catalysts, imidodiphosphorimidates (IDPis), which have a rather high acidity and a confined chiral microenvironment [58]. In 2017, List et al. reported an asymmetric hetero-Diels–Alder reaction between dienes and aldehydes to furnish multi-substituted dihydropyrans with excellent enantioselectivity [59] (Scheme 3a). This method was successfully applied for the efficient synthesis of the commercialized fragrances Verdirosa and Pelargene. Doremox, a rose oxide replacement fragrance, can be also obtained via the diastereoselective hydrogenation of Verdirosa. More importantly, this method enables researchers to build a library of diverse and enantiomerically enriched dihydropyrans and tetrahydropyrans in a modular fashion, which may accelerate the discovery of new fragrances and promote olfactory understanding of this scaffold.
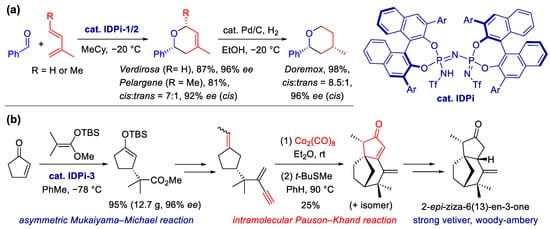
Scheme 3. Fragrance synthesis by the List group. (a) Synthesis of hydropyran fragrances via the asymmetric hetero-Diels–Alder reaction, catalyzed by imidodiphosphorimidate (IDPi). (b) Total synthesis of the principal ingredient of vetiver oil with IDPi-catalyzed asymmetric Mukaiyama–Michael addition and Co2(CO)8-mediated Pauson–Khand [2 + 2 + 1] cyclization as the key steps.
With a time-honored and worldwide utilization in a range of perfumes, including Chanel N° 5, vetiver oil represents one of the most well-known perfumery materials, but the molecular origin of its special odor remains unclear. In 2021, the List group elucidated the fragrant principle of vetiver oil in collaboration with the Kraft group at Givaudan [60]. With total synthesis and olfactory study of (+)-2-epi-ziza-6(13)en-3-one (Scheme 3b), they proved this is the key scent contributor to the characteristic transparent woody–ambery odor of vetiver oil. This compound has a featured and potent vetiver odor with a remarkable threshold of 29 pg/L air. Notably, its structure superimposes quite well on that of arborone in stereochemical superposition analysis, which partly revealed the molecular mechanism of their similar transparent woody–ambery notes and the “magic quasi-pheromone-like” effect.
The synthetic route was initiated by an asymmetric Mukaiyama–Michael addition [61], which is catalyzed by the IDPi on a multigram scale with an impressive 0.1 mol% of catalyst loading. After establishing the original enantioselectivity, the adduct was transformed into an enyne intermediate in several steps. The following intramolecular [2 + 2 + 1] cycloaddition is another key step, which was expected to form the desired polycyclic scaffold with sterically well-arranged substituents. However, this challenging cyclization cannot proceed under common Pauson–Khand conditions. With the compromise of atom and step economies, a stepwise protocol was finally employed. Stoichiometric Co2(CO)8 was needed to form an isolatable cobalt complex that was then treated with excess tert-butyl (methyl)sulfane to furnish the cyclization. Although the yield and selectivity were not high, the Pauson–Khand reaction accomplished the goal of rapid construction of the challenging scaffold; the entire synthetic route is still efficient enough when compared to the isolation of a single enantiopure ingredient from hundreds of sesquiterpenoid constituents in vetiver oil.
Musky fragrances are omnipresent and indispensable in perfumery and exploring new types of musks is a continuous goal in fragrance chemistry. In 2013, Tacke and Kraft et al. synthesized the silicon-containing analogs of galaxolide [62] (Scheme 4a), a polycyclic musk odorant for more than half a century. A Co-catalyzed [2 + 2 + 2] cycloaddition of the disila-diynes with a multifunctional alkyne was first conducted to construct a benzene ring with well-arranged functional groups, a strategy previously used for the construction of several disila-analogs of the well-known musk, phantolide [63]. After the conversion of the borate group to bromide, an intramolecular Heck cross-coupling reaction was performed. The desired products with an exocyclic double bond were obtained, together with two separatable regioisomers; then, they underwent hydrogenation to deliver sila-analogs of galaxolide. Furthermore, the corresponding diastereomers can be obtained from the racemates by the formation–decomplexation of chromatographically separatable Cr-complexes. This work exemplified the sequential transformation of alkynes and alkenes to enable the synthesis of polycyclic fragrances, but selectivity and step economy still have plenty of room for improvement. An enantioselective reductive Heck reaction may be a possible strategy to meet this challenge [64,65].
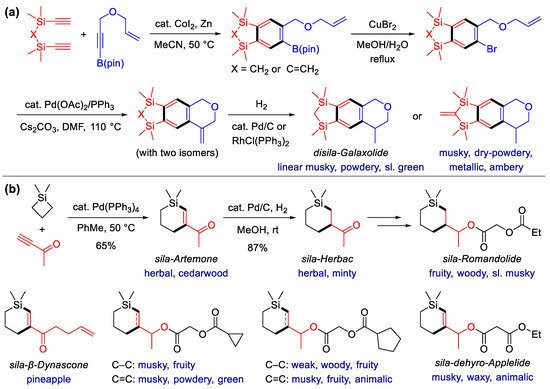
Scheme 4. Synthesis of Si-heterocyclic fragrances via alkyne annulation. (a) Synthesis of sila-analogs of galaxolide with Co-catalyzed [2 + 2 + 2] alkyne cycloaddition as the key step; (b) synthesis of fragrant six-membered silacycles via the Pd-catalyzed [4 + 2] reaction of silacyclobutanes and alkynes.
In 2014, Liu et al. reported an efficient and regioselective Pd(0)-catalyzed [4 + 2] cyclization for the construction of a six-membered silacycle from silacyclobutanes and terminal alkynes [66] (Scheme 4b). The reaction is proposed to be initiated by the oxidative insertion of the Pd(0) species to the silacyclobutane ring, to form a five-membered palladasilacycle, which was coordinated with alkyne, with regioselectivity controlled by steric hindrance. After alkyne insertion, a seven-membered palladasilacycle is formed, which undergoes reductive elimination to yield the resulting product. This method was successfully applied for the synthesis of sila-analogs of Artemone, Herbac and β-Dynascone for commercial fragrances produced by Givaudan, IFF and Firmenich, respectively. Sila-Artemone was obtained first and was then converted to Sila-Herbac via hydrogenation. Sila-β-Dynascone can be synthesized either via the derivation of sila-Artemone, or via direct cyclization using hept-6-en-1-yn-3-one as the alkyne partner.
In a subsequent work in 2016, Liu et al. further synthesized a series of ester-containing silacycles starting from sila-Artemone [67]. Besides the sila-analogs of linear alicyclic musks such as Rosamusk, Romandolide and Applelide, analogs with cyclopropyl or cyclopentyl were also synthesized (Scheme 4b). Notably, some of the sila-analogs have a dominant fruity and woody odor instead of a musky character. After studying the odor character, thresholds and activity value, the structure–odor correlations of these Si-containing odorants were also analyzed. On this basis, a refined musk olfactophore model was proposed, which indicates that linear and macrocyclic musks may address the same odorant receptors.
Besides π reactants and strained rings, carbenes are also a widely used component of cyclization. The cyclopropanation of alkenes using carbenes, which can be treated as a type of [2 + 1] cyclization, has been widely used for the synthesis of natural products and fragrances [31,63,68,69,70,71]. In 2016, Baldovini et al. reported an elegant total synthesis of four isomers of olibanic acids, with the key steps involved in the cis/trans-selective semi-hydrogenation of alkynes and stereodivergent asymmetric cyclopropanation [69] (Scheme 5a). Facilitated by this synthesis, they further revealed that two of them are key odorants responsible for the characteristic notes of frankincense. Carbenes can also be reacted with dienes to facilitate [4 + 1] cyclization. A representative example was demonstrated by Spino et al. for the total synthesis of carotol [72], a sesquiterpene identified as one of the major ingredients in carrot seed essential oil [73,74]. The synthetic route was initiated with an intramolecular formal [4 + 1] cycloaddition of the dialkoxycarbene and electron-deficient diene, which can also proceed smoothly using a substrate with a chiral auxiliary (Scheme 5b). After several steps involving ring-opening, functionalization and transformations, a ring-closing olefin metathesis was processed to yield carotol.
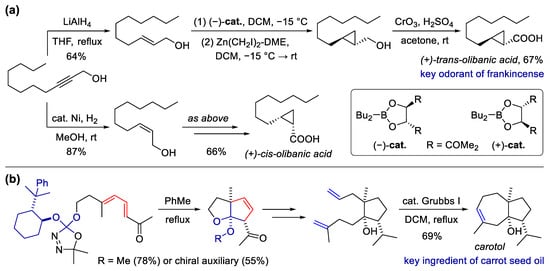
Scheme 5. Synthesis of fragrant natural products using carbene-involved cyclization. (a) Total synthesis of olibanic acids via semi-hydrogenation and cyclopropanation; (b) total synthesis of carotol via [4 + 1] cyclization and ring-closing metathesis.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27113576
This entry is offline, you can click here to edit this entry!
