The Mueller matrix (4 × 4) most fully describes the interaction of an arbitrary object with fully or partially polarized electromagnetic radiation; therefore, the existing polarimetric methods can be generalized by the Mueller matrix formalism, and that generalization is termed Mueller matrix polarimetry (MMP). Mueller matrix measurements, being integrated into standard schemes of conventional optical methods, such as scatterometry, optical coherence tomography, fluorimetry, spectrophotometry and reflectometry, can significantly expand their capabilities in the characterization of biological systems and bioorganic materials.
- Mueller matrix polarimetry
- scatterometry
- spectropolarimetry
- OCT
- fluorimetry
- ellipsometry
- biology
- agriculture
1. Introduction
2. Principles of Mueller Matrix Analysis


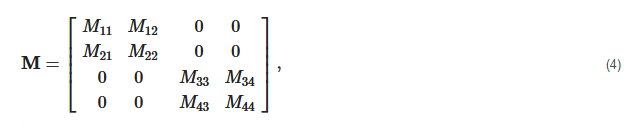
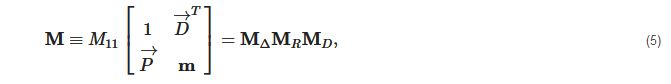
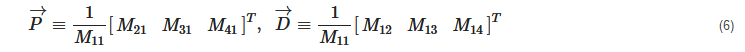

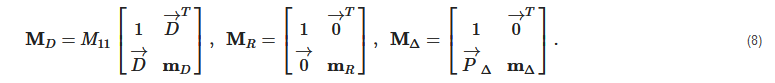

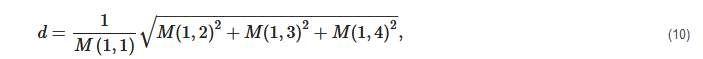

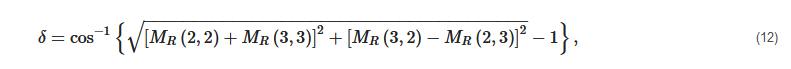
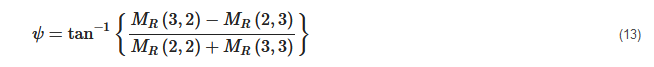
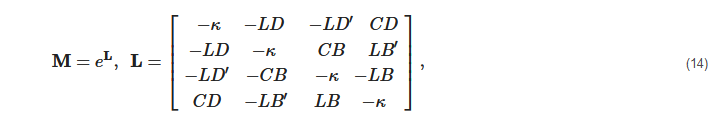

- Polarization is modulated continuously or pulse-modulated (periodically, as a rule);
- Polarization sequentially passes a fixed, discrete set of time-constant states.

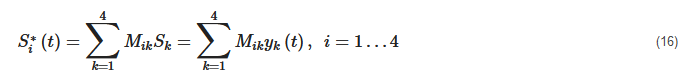

3. Basic Schemes for Using Mueller Matrix Polarimetry in Optical Diagnostics of Biological and Bioorganic Systems
4. Applications of MMP to Biology and Agriculture
- Geometry of the sample—volumetric (MM scatterometry, MM spectrophotometry, MM fluorometry) and surface (MM-OCT and MM ellipsometry);
- Properties of incoming radiation—spectral (spectrophotometry and spectroscopic MM scatterometry, MM fluorometry and MM ellipsometry) and non-spectral (MM-OCT and single-wavelength MM scatterometry, MM fluorometry and MM ellipsometry);
- Received information—visual (MM-OCT and other methods of polarization-sensitive imaging) and structural (all methods that measure the MM with subsequent retrieval of the microphysical parameters of the sample using inverse analysis);
- Operating conditions—stationary and mobile.
4.1. Studying the Structure of Organic Materials
4.2. Microbiological Research
4.3. Biological Tissues
4.4. Milk Composition Analysis and Food Quality Control
4.5. Characterization of Soils and Fodders and Vegetation Monitoring
4.6. Further Development of MMP Diagnostics Using Fiber Optics
This entry is adapted from the peer-reviewed paper 10.3390/app12105258
References
- Fuller, G.G. Optical Rheometry of Complex Fluids; Oxford University Press: New York, NY, USA, 1995.
- Palberg, T.; Ballauff, M. Optical Methods and Physics of Colloidal Dispersions. In Proceedings of the International Workshop on Optical Methods and the Physics of Colloidal Dispersions held in Memory of Prof. Dr. Klaus Schätzel, Mainz, Germany, 30 September 1996; Steinkopff: Heidelberg, Germany, 1997.
- Xu, R. Particle Characterization: Light Scattering Methods; Kluwer Academic Publishers: New York, NY, USA, 2002.
- Mishchenko, M.I.; Travis, L.D.; Lacis, A.A. Scattering, Absorption, and Emission of Light by Small Particles; Cambridge University Press: Cambridge, UK, 2002.
- Doicu, A.; Wriedt, T.; Eremin, Y.A. Light Scattering by Systems of Particles; Springer: Berlin/Heidelberg, Germany, 2006.
- Azzam, R.M. Stokes-vector and Mueller-matrix polarimetry. J. Opt. Soc. Am. A 2016, 33, 1396–1408.
- Chipman, R.A.; Sornsin, E.A.; Pezzaniti, J.L. Mueller matrix imaging polarimetry: An overview. In Proceedings of the SPIE, International Symposium on Polarization Analysis and Applications to Device Technology, Yokohama, Japan, 12–16 June 1996; Yoshizawa, T., Yokota, H., Eds.; SPIE: Bellingham, WA, USA, 1996; Volume 2873, pp. 5–12.
- Arteaga, O.; Kahr, B. Mueller matrix polarimetry of bianisotropic materials. J. Opt. Soc. Am. B 2019, 36, F72–F83.
- Ghosh, N.; Soni, J.; Wood, M.; Wallenberg, M.; Vitkin, I. Mueller matrix polarimetry for the characterization of complex random medium like biological tissues. Pramana J. Phys. 2010, 75, 1071–1086.
- Tripathi, S.; Toussaint, K.C. Rapid Mueller matrix polarimetry based on parallelized polarization state generation and detection. Opt. Express 2009, 17, 21396–21407.
- Anastasiadou, M.; Hatit, S.B.; Ossikovski, R.; Guyot, S.; De Martino, A. Experimental validation of the reverse polar decomposition of depolarizing Mueller matrices. J. Eur. Opt. Soc. Rapid Publ. 2007, 2, 07018.
- Arwin, H.; Schoeche, S.; Hilfiker, J.; Hartveit, M.; Järrendahl, K.; Juárez-Rivera, O.R.; Mendoza-Galván, A.; Magnusson, R. Optical Chirality Determined from Mueller Matrices. Appl. Sci. 2021, 11, 6742.
- Boulvert, F.; Le Brun, G.; Le Jeune, B.; Cariou, J.; Martin, L. Decomposition algorithm of an experimental Mueller matrix. Opt. Commun. 2009, 282, 692–704.
- Liu, J.; Zhang, Q.; Huo, Y.; Wang, J.; Zhang, Y. An experimental study on light scattering matrices for Chinese loess dust with different particle size distributions. Atmos. Meas. Tech. 2020, 13, 4097–4109.
- Volten, H.; Jalava, J.P.; Lumme, K.; De Haan, J.F.; Vassen, W.; Hovenier, J.W. Laboratory measurements and T-matrix calculations of the scattering matrix of rutile particles in water. Appl. Opt. 1999, 38, 5232–5240.
- Diaspro, A.; Radicchi, G.; Nicolini, C. Polarized light scattering: A biophysical method for studying bacterial cells. IEEE Trans. Biomed. Eng. 1995, 42, 1038–1043.
- Mengüç, M.; Manickavasagam, S. Characterization of size and structure of agglomerates and inhomogeneous particles via polarized light. Int. J. Eng. Sci. 1998, 36, 1569–1593.
- Kolokolova, L.; Kimura, H.; Ziegler, K.; Mann, I. Light-scattering properties of random-oriented aggregates: Do they represent the properties of an ensemble of aggregates? J. Quant. Spectrosc. Radiat. Transf. 2006, 100, 199–206.
- Aiello, A.; Woerdman, J. Linear Algebra for Mueller Calculus. arXiv 2004, arXiv:Math-ph/0412061. Available online: https://arxiv.org/abs/math-ph/0412061 (accessed on 17 December 2004).
- Gil, J.J. Review on Mueller matrix algebra for the analysis of polarimetric measurements. J. Appl. Rem. Sens. 2014, 8, 081599.
- Ding, H.; Lu, J.Q.; Brock, R.S.; McConnell, T.J.; Ojeda, J.F.; Jacobs, K.M.; Hu, X.H. Angle-resolved Mueller matrix study of light scattering by B-cells at three wavelengths of 442, 633, and 850 nm. J. Biomed. Opt. 2007, 12, 034032.
- Van de Merwe, W.; Li, Z.Z.; Bronk, B.V.; Czégé, J. Polarized light scattering for rapid observation of bacterial size changes. Biophys. J. 1997, 73, 500–506.
- Le Gratiet, A.; Marongiu, R.; Diaspro, A. Circular Intensity Differential Scattering for Label-Free Chromatin Characterization: A Review for Optical Microscopy. Polymers 2020, 12, 2428.
- Pan, Y.L.; Aptowicz, K.; Arnold, J.; Cheng, S.; Kalume, A.; Piedra, P.; Wang, C.; Santarpia, J.; Videen, G. Review of elastic light scattering from single aerosol particles and application in bioaerosol detection. J. Quant. Spectrosc. Radiat. Transf. 2022, 279, 108067.
- Neuman, M. Angle Resolved Light Scattering in Turbid Media: Analysis and Applications. Ph.D. Thesis, Mid Sweden University, Härnösand, Sweden, 2011. Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:miun:diva-13154 (accessed on 25 January 2011).
- Ghosh, N.; Vitkin, I.A. Tissue polarimetry: Concepts, challenges, applications, and outlook. J. Biomed. Opt. 2011, 16, 110801.
- Fanjul-Velez, F.; Samperio-Garcia, D.; Pereda-Cubian, D.; Arce-Diego, J.L. Mueller matrix group theory Formalism for tissue imaging polarimetry contrast increase. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007.
- Ramella-Roman, J.C.; Prahl, S.A.; Jacques, S.L. Three Monte Carlo programs of polarized light transport into scattering media: Part I. Opt. Express 2005, 13, 4420–4438.
- Ramella-Roman, J.C.; Prahl, S.A.; Jacques, S.L. Three Monte Carlo programs of polarized light transport into scattering media: Part II. Opt. Express 2005, 13, 10392–10405.
- Wang, X.; Yao, G.; Wang, L.V. Monte Carlo model and single-scattering approximation of the propagation of polarized light in turbid media containing glucose. Appl. Opt. 2002, 41, 792–801.
- Wang, L.F. Monte Carlo Simulation Model for Electromagnetic Scattering from Vegetation and Inversion of Vegetation Parameters. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2007. Available online: http://hdl.handle.net/1721.1/38923 (accessed on 28 September 2007).
- Tuchin, V.V. Polarized light interaction with tissues. J. Biomed. Opt. 2016, 21, 071114.
- Tuchin, V.V.; Zhu, D.; Genina, E.A. (Eds.) Handbook of Tissue Optical Clearing: New Prospects in Optical Imaging; CRC Press: Boca Raton, FL, USA, 2022.
- Lu, S.; Chipman, R. Interpretation of Mueller matrices based on polar decomposition. J. Opt. Soc. Am. A 1996, 13, 1106–1113.
- Shindo, Y.; Oda, Y.; Oshima, A.; Maeda, S. New type of CD spectropolarimeter with LD option. Rev. Sci. Instrum. 1993, 64, 1161–1168.
- Garcia-Caurel, E.; De Martino, A.; Drévillon, B. Spectroscopic Mueller polarimeter based on liquid crystal devices. Thin Solid Film 2004, 455–456, 120–123.
- Dubreuil, M.; Rivet, S.; Le Jeune, B.; Cariou, J. Snapshot Mueller matrix polarimeter by wavelength polarization coding. Opt. Express 2007, 15, 13660–13668.
- Protsenko, E.D.; Tymper, S.I.; Shkirin, A.V. Automated laser IR spectropolarimeter for surface Mueller matrix measurements. Instrum. Exp. Tech. 2011, 51, 268–274.
- Bueno, J.M. Polarimetry using liquid-crystal variable retarders: Theory and calibration. J. Opt. A Pure Appl. Opt. 2000, 2, 216–222.
- Suárez-Bermejo, J.C.; de Sande, J.C.G.; Santarsiero, M.; Piquero, G. Mueller matrix polarimetry using full Poincaré beams. Opt. Lasers Eng. 2019, 122, 134–141.
- Arteaga, O.; Freudenthal, J.; Wang, B.; Kahr, B. Mueller matrix polarimetry with four photoelastic modulators: Theory and calibration. Appl. Opt. 2012, 51, 6805–6817.
- Goldstein, D.H.; Chipman, R.A. Error analysis of a Mueller matrix polarimeter. J. Opt. Soc. Am. A 1990, 7, 693–700.
- Swamy, J.N.; Crofcheck, C.; Mengüç, M.P. Time dependent scattering properties of slow decaying liquid foams. Colloids Surf. A Physicochem. Eng. Asp. 2009, 338, 80–86.
- Laskarakis, A.; Logothetidis, S.; Pavlopoulou, E.; Gioti, M. Mueller matrix spectroscopic ellipsometry: Formulation and application. Thin Solid Films 2004, 455, 43–49.
- Mendoza-Galván, A.; Muñoz-Pineda, E.; Ribeiro, S.J.L.; Santos, M.V.; Järrendahl, K.; Arwin, H. Mueller matrix spectroscopic ellipsometry study of chiral nanocrystalline cellulose films. J. Opt. 2018, 20, 024001.
- Hilfiker, J.; Herzinger, C.; Wagner, T.; Marino, A.; Delgais, G.; Abbate, G. Mueller-matrix characterization of liquid crystals. Thin Solid Films 2004, 455, 591–595.
- Kupinski, M.; Li, L. Evaluating the Utility of Mueller Matrix Imaging for Diffuse Material Classification. J. Imaging Sci. Technol. 2020, 64, 60409.
- Juárez-Rivera, O.R.; Mauricio-Sánchez, R.A.; Järrendahl, K.; Arwin, H.; Mendoza-Galván, A. Shear-Coated Linear Birefringent and Chiral Cellulose Nanocrystal Films Prepared from Non-Sonicated Suspensions with Different Storage Time. Nanomaterials 2021, 11, 2239.
- Mendoza-Galván, A.; Li, Y.; Yang, X.; Magnusson, R.; Järrendahl, K.; Berglund, L.; Arwin, H. Transmission Mueller-matrix characterization of transparent ramie films. J. Vac. Sci. Technol. B 2020, 38, 014008.
- Fricke, D.; Becker, A.; Jutte, L.; Bode, M.; de Cassan, D.; Wollweber, M.; Glasmacher, B.; Roth, B. Mueller Matrix Measurement of Electrospun Fiber Scaffolds for Tissue Engineering. Polymers 2019, 11, 2062.
- Chen, C.; Chen, X.; Shi, Y.; Gu, H.; Jiang, H.; Liu, S. Metrology of Nanostructures by Tomographic Mueller-Matrix Scatterometry. Appl. Sci. 2018, 8, 2583.
- Deibler, L.L.; Smith, M.H. Measurement of the complex refractive index of isotropic materials with Mueller matrix polarimetry. Appl. Opt. 2001, 40, 3659–3667.
- Furchner, A.; Walder, C.; Zellmeier, M.; Rappich, J.; Hinrichs, K. Broadband infrared Mueller-matrix ellipsometry for studies of structured surfaces and thin films. Appl. Opt. 2018, 57, 7895–7904.
- Furchner, A.; Kratz, C.; Ogieglo, W.; Pinnau, I.; Rappich, J.; Hinrichs, K. Ultrasensitive broadband infrared 4 × 4 Mueller-matrix ellipsometry for studies of depolarizing and anisotropic thin films. J. Vac. Sci. Technol. B 2020, 38, 014003.
- Den Boer, J.H.W.G. Spectroscopic Infrared Ellipsometry: Components, Calibration, and Application; Technische Universiteit: Eindhoven, The Netherlands, 1995.
- Bunkin, N.F.; Shkirin, A.V.; Ninham, B.W.; Chirikov, S.N.; Chaikov, L.L.; Penkov, N.V.; Kozlov, V.A.; Gudkov, S.V. Shaking-Induced Aggregation and Flotation in Immunoglobulin Dispersions: Differences between Water and Water–Ethanol Mixtures. ACS Omega 2020, 5, 14689–14701.
- Lundblad, J.R.; Laurance, M.; Goodman, R.H. Fluorescence polarization analysis of protein-DNA and protein-protein interactions. Mol. Endocrinol. 1996, 10, 607–612.
- Maji, K.; Saha, S.; Dey, R.; Ghosh, N.; Haldar, D. Mueller Matrix Fluorescence Spectroscopy for Probing Self-Assembled Peptide-Based Hybrid Supramolecular Structure and Orientation. J. Phys. Chem. C 2017, 121, 19519–19529.
- Thermo Fisher Scientific. Molecular Probes Handbook. Fluorescence Polarization (FP). Available online: https://www.thermofisher.com/ru/ru/home/references/molecular-probes-the-handbook/technical-notes-and-product-highlights/fluorescence-polarization-fp.html (accessed on 18 July 2017).
- He, C.; He, H.; Chang, J.; Chen, B.; Ma, H.; Booth, M.J. Polarisation optics for biomedical and clinical applications: A review. Light Sci. Appl. 2021, 10, 194.
- Svensen, Ø.; Stamnes, J.; Kildemo, M.; Aas, L.; Erga, S.; Frette, Ø. Mueller matrix measurements of algae with different shape and size distributions. Appl. Opt. 2011, 50, 5149–5157.
- Badieyan, S.; Dilmaghani-Marand, A.; Hajipour, M.J.; Ameri, A.; Razzaghi, M.R.; Rafii-Tabar, H.; Mahmoudi, M.; Sasanpour, P. Detection and Discrimination of Bacterial Colonies with Mueller Matrix Imaging. Sci. Rep. 2018, 8, 10815.
- Chami, M. Importance of the polarization in the retrieval of oceanic constituents from the remote sensing reflectance. J. Geophys. Res. 2007, 112, C05026.
- Badieyan, S.; Ameri, A.; Razzaghi, M.R.; Rafii-Tabar, H.; Sasanpour, P. Mueller matrix imaging of prostate bulk tissues; Polarization parameters as a discriminating benchmark. Photodiagnosis Photodyn. Ther. 2019, 26, 90–96.
- Du, E.; He, H.; Zeng, N.; Sun, M.; Guo, Y.; Wu, J.; Liu, S.; Ma, H. Mueller matrix polarimetry for differentiating characteristic features of cancerous tissues. J. Biomed. Opt. 2014, 19, 076013.
- Liu, T.; Sun, T.; He, H.; Liu, S.; Dong, Y.; Wu, J.; Ma, H. Comparative study of the imaging contrasts of Mueller matrix derived parameters between transmission and backscattering polarimetry. Biomed. Opt. Express 2018, 9, 4413–4428.
- Wu, X.; Calabia, A.; Xu, J.; Bai, W.; Guo, P. Forest canopy scattering properties with signal of opportunity reflectometry: Theoretical simulations. Geosci. Lett. 2021, 8, 25.
- Ahmad, I.; Khaliq, A.; Iqbal, M.; Khan, S. Mueller matrix polarimetry for characterization of skin tissue samples: A review. Photodiagnosis Photodyn. Ther. 2020, 30, 101708.
- Smith, M.H.; Burke, P.D.; Lompado, A.; Tanner, E.A.; Hillman, L.W. Mueller matrix imaging polarimetry in dermatology. In Proceedings of the SPIE 3911, Biomedical Diagnostic, Guidance, and Surgical-Assist Systems II, San Jose, CA, USA, 3 May 2000.
- Jiao, S.; Yu, W.; Stoica, G.; Wang, L.V. Multiple-channel Mueller-matrix optical coherence tomography in biological tissue. In Proceedings of the Second Joint 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society, Houston, TX, USA, 23–26 October 2002; Volume 3, pp. 2321–2322.
- Borovkova, M.; Trifonyuk, L.; Ushenko, V.; Dubolazov, O.; Vanchulyak, O.; Bodnar, G.; Ushenko, Y.; Olar, O.; Ushenko, O.; Sakhnovskiy, M.; et al. Mueller-matrix-based polarization imaging and quantitative assessment of optically anisotropic polycrystalline networks. PLoS ONE 2019, 14, e0214494.
- Ding, Z.; Liang, C.P.; Tang, Q.; Chen, Y. Quantitative single-mode fiber based PS-OCT with single input polarization state using Mueller matrix. Biomed. Opt. Express 2015, 6, 1828–1843.
- Guo, X.; Wood, M.; Vitkin, A.I. Angular measurements of light scattered by turbid chiral media using linear Stokes polarimeter. J. Biomed. Opt. 2006, 11, 041105.
- Ahmad, I.; Gribble, A.; Murtza, I.; Ikram, M.; Pop, M.; Vitkin, A. Polarization image segmentation of radiofrequency ablated porcine myocardial tissue. PLoS ONE 2017, 12, e0175173.
- Jagtap, J.; Chandel, S.; Das, N.; Soni, J.; Chatterjee, S.; Pradhan, A.; Ghosh, N. Quantitative Mueller matrix fluorescence spectroscopy for precancer detection. Opt. Lett. 2014, 39, 243–246.
- Soni, J.; Purwar, H.; Lakhotia, H.; Chandel, S.; Banerjee, C.; Kumar, U.; Ghosh, N. Quantitative fluorescence and elastic scattering tissue polarimetry using an Eigenvalue calibrated spectroscopic Mueller matrix system. Opt. Express 2013, 21, 15475–15489.
- Sheng, W.; Li, W.; Qi, J.; Liu, T.; He, H.; Dong, Y.; Liu, S.; Wu, J.; Elson, D.S.; Ma, H. Quantitative Analysis of 4 × 4 Mueller Matrix Transformation Parameters for Biomedical Imaging. Photonics 2019, 6, 34.
- He, H.; Zeng, N.; Li, D.; Liao, R.; Ma, H. Quantitative Mueller matrix polarimetry techniques for biological tissues. J. Innov. Opt. Health Sci. 2012, 5, 1250017.
- Firdous, S. Polarization Sensitive Optical Imaging and Characterization of Soybean Using Stokes-Mueller Matrix Model. In Soybean—Genetics and Novel Techniques for Yield Enhancement; Krezhova, D., Ed.; IntechOpen: London, UK, 2011.
- Veenstra, C.; Every, D.E.; Petersen, W.; van Goudoever, J.B.; Steenbergen, W.; Bosschaart, N. Dependency of the optical scattering properties of human milk on casein content and common sample preparation methods. J. Biomed. Opt. 2020, 25, 045001.
- Crofcheck, C.; Wade, J.; Swamy, J.N.; Aslan, M.M.; Mengüç, M.P. Effect of Fat and Casein Particles in Milk on the Scattering of Elliptically Polarized Light. Trans. ASAE 2005, 48, 1147–1155.
- Shkirin, A.V.; Ignatenko, D.N.; Chirikov, S.N.; Bunkin, N.F.; Astashev, M.E.; Gudkov, S.V. Analysis of Fat and Protein Content in Milk Using Laser Polarimetric Scatterometry. Agriculture 2021, 11, 1028.
- Lamelas, F.; Swaminathan, S. Optical absorption, scattering, and multiple scattering: Experimental measurements using food coloring, India ink, and milk. Am. J. Phys. 2020, 88, 137–140.
- Lehmann, P.; Osten, W.; Albertazzi Gonçalves, A.; Jain, P.; Sarma, S.E. Light scattering and transmission measurement using digital imaging for online analysis of constituents in milk. In Proceedings of the Optical Measurement Systems for Industrial Inspection, Munich, Germany, 21 June 2015.
- Derman, D.; Şenel, E.C.; Opar, E.; Ferhanoğlu, O.; Polat, Ö. Optical characterization of olive and sun flower oils via Mueller matrix polarimetry in combination with principal component analysis. J. Food Meas. Charact. 2021, 15, 2309–2317.
- Peyvasteh, M.; Popov, A.; Bykov, A.; Pierangelo, A.; Novikova, T.; Meglinski, I. Evolution of raw meat polarization-based properties by means of Mueller matrix imaging. J. Biophotonics 2021, 14, e202000376.
- Bunkin, N.F.; Glinushkin, A.P.; Shkirin, A.V.; Ignatenko, D.N.; Chirikov, S.N.; Savchenko, I.V.; Meshalkin, V.P.; Samarin, G.N.; Maleki, A.; Kalinitchenko, V.P. Identification of Organic Matter Dispersions Based on Light Scattering Matrices Focusing on Soil Organic Matter Management. ACS Omega 2020, 5, 33214–33224.
- Oh, Y.; Sarabandi, K.; Ulaby, F.T. Semi-empirical model of the ensemble-averaged differential Mueller matrix for microwave backscattering from bare soil surfaces. IEEE Trans. Geosci. Remote Sens. 2002, 40, 1348–1355.
- Zallat, J.; Stoll, M.P. Polarized bidirectional scattering by bare soils. J. Opt. A Pure Appl. Opt. 2000, 2, 169.
- Jin, Y.Q. Theoretical Modeling for Polarimetric Scattering and Information Retrieval of SAR Remote Sensing. In Advances in Geoscience and Remote Sensing; IntechOpen: London, UK, 2009.
- Jiao, S.; Yu, W.; Stoica, G.; Wang, L. Optical-fiber-based Mueller optical coherence tomography. Opt. Lett. 2003, 28, 1206–1208.
- Dong, H.; Shum, P.; Gong, Y.D.; Yan, M.; Zhou, J.Q.; Wu, C.Q. Virtual Generalized Mueller Matrix Method for Measurement of Complex Polarization-Mode Dispersion Vector in Optical Fibers. IEEE Photonics Technol. Lett. 2007, 19, 27–29.
- Dong, H.; Shum, P.; Yan, M.; Zhou, J.Q.; Ning, G.X.; Gong, Y.D.; Wu, C.Q. Measurement of Mueller matrix for an optical fiber system with birefringence and polarization-dependent loss or gain. Opt. Commun. 2007, 274, 116–123.
- Qi, J.; Elson, D.S. Mueller polarimetric imaging for surgical and diagnostic applications: A review. J. Biophotonics 2017, 10, 950–982.
