Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Hematology
Chimeric antigen receptor T (CAR T) cell therapy is promising for relapse/refractory chronic lymphocytic leukemia (CLL) patients. Complete and durable remission of CLL is possible in patients treated with CAR T cells but further investigations are necessary to understand and possibly predict how patient specific factors influence the outcome of this treatment.
- CAR T cells
- CAR NK cells
- CLL
1. Introduction
Chronic lymphocytic leukemia (CLL) is a chronic lymphoproliferative disease characterized by malignant transformation of mature antigen-experienced B lymphocyte and accumulation of monoclonal malignant B cells in peripheral blood, bone marrow, lymph nodes, spleen [1]. CLL is the most common leukemia in Western countries. The incidence of CLL in North America and Eastern Europe is 4.7 per 100,000 people per year, while the CLL incidence is higher than 35 in the population over 85 years of age [2]. Clinical management of CLL is challenging and depends on patients ages, comorbidities and biological features of CLL cells such as immunoglobulin heavy chain gene mutation, 17p deletion, TP53 mutation and, as recent research showed, number and type of CLL tumor clones in patient [3,4]. Treatment option varies from watch and wait approach in the asymptomatic early-stage CLL to chemo-immunotherapy and novel targeted therapies, such as Bruton’s tyrosine kinase inhibitors and inhibitors of Bcl-2 family proteins, for symptomatic and advanced disease [3]. However, despite the expansion of novel therapeutic approaches, CLL is still mostly an incurable disease.
In the last decades, cell-based immunotherapy has emerged as a novel treatment for malignant diseases. Cell based immunotherapy relies on using immune cells obtained from patients, raised in vitro, and genetically modified to increase their ability to find and kill tumor cells (Figure 1). Various T cell based treatments of malignant disorders have been developed, including tumor-infiltrating lymphocytes, T cell receptor (TCR)-modified T cells and chimeric antigen receptor T (CAR T) cells [5]. Therapy of malignancies with CAR T cells is accompanied with better and durable clinical responses compared to the treatment with tumor-infiltrating lymphocytes and TCR modified T cells [6,7]. CAR T cells are T lymphocytes with engineered synthetic receptors made to recognize and destroy the cells expressing the target antigen. CARs are artificially made proteins consisting of the single-chain variable fragment of an antibody (ScFv) that recognizes tumor antigen and the T-cell activation domain [8].
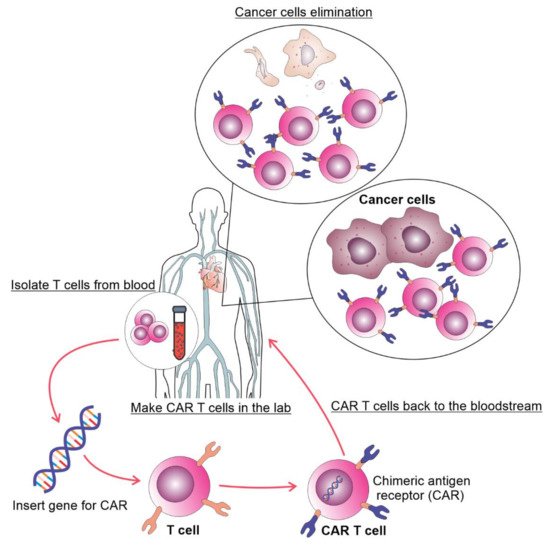
Figure 1. The process of autologous CAR T cell therapy. T cells are collected via apheresis, genetically reengineered in a laboratory by introducing DNA that encodes CAR, multiplied, and then infused into the patient. The CAR T cells may eliminate all of the cancer cells and may remain in the body months after the infusion.
According to the T-cell activation domain, which is part of the hybrid receptor, CAR T cells are classified into four generations. The first generation of CAR T cells contains only CD3 zeta domain in fusion with receptors specific for a specified tumor antigen. These first generation CAR T cells showed limited clinical effects. Hybrid receptors of the second generation of CAR T cells in addition to CD3 contain one of the additional costimulatory molecules such as CD28, ICOS, CD-137/4-1BB or OX40, while coupling two or more costimulatory molecules into the receptor sequence led to the creation of the third generation CAR T cells [8]. The fourth CAR T cells’ generation is engineered with the inducible expression of cytokines that potentiate antitumor immunity and a self-withdrawal mechanism, with a suicide gene that can be activated after the achievement of the anti-tumor effect whose product rapidly withdraws CAR T cells [9]. The good clinical responses to CAR T cell therapy in some malignancies are frequently accompanied by several obstacles (Figure 2). Since most of the patients are highly medicated resulting in a low number and quality of T cells, manufacturing and expanding autologous CAR T cells from such patients can be difficult [10]. The manufacture of allogenic CAR T cells from healthy donors is promising but allogenic CAR T cells can cause a serious graft-versus-host disease (GvHD) [11]. However, the fact that allogenic NK cells have reduced the risk for induction of GvDH led to the construction of CAR Natural Killer (NK) cells. CAR constructs in CAR NK cells play the role in the NK cell activation and also improve the efficiency of innate ability of NK cells to kill malignant cells [12].
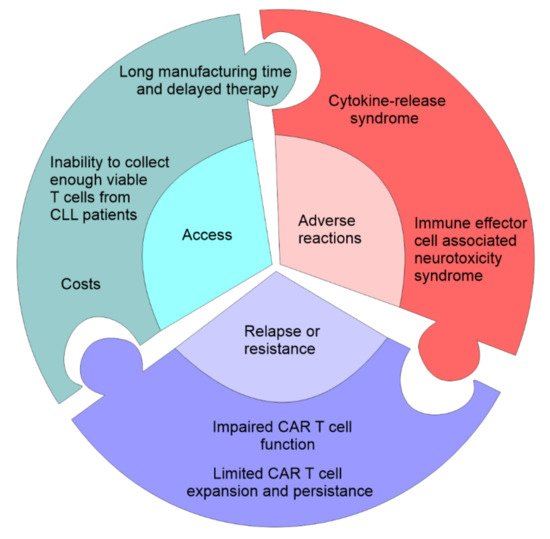
Figure 2. Challenges in CAR T-cell therapy. The first problem associated with CAR T cell therapy is access which can be limited by the cost of the manufacture of CAR T cells, time needed to manufacture and expand CAR T cells, failure to produce autologous CAR T cells, and eligibility for the clinical trial. The second problem associated with CAR T cell therapy are serious adverse event: cytokine-release storm (widespread pyroptosis of tumor cells is followed by the release of different factors that activate macrophages to produce inflammatory cytokines that induce systemic inflammatory response), and neurotoxicity syndrome (toxic encephalopathy caused by the disruption of the blood-brain-barrier). CAR T cell therapy may be accompanied by the primary resistance due to the impaired CAR T cell function and the inability to induce remission and limited CAR T cell expansion.
Although the use of cellular immunotherapy in many hematologic malignancies is already approved outside clinical trials [13,14,15,16], the benefit of CAR T and CAR NK cells in CLL treatment is still not clear.
2. CAR-T as Promising CLL Therapy
In the early stage of development of CAR-T cells for B cell leukemia, CD19 has become attractive target antigen because it is expressed on the most B-cell malignancies [17]. Results of preclinical studies indicate better in vivo expansion and far greater effectiveness of the second generation of CAR-T cells, with CARs constructed to target CD19 coupled with CD137 signaling or CD28 costimulatory domain, in comparison to the first generation of CAR T cells [18,19]. The first use of CAR T cells in the therapy of CLL was reported in 2011 [20]. Porter et al. reported the treatment of two patients with refractory/relapsed CLL with reinfusion of approximately 1.5 × 105 autologous CAR T cells per kilogram of body weight that expanded to a level that was more than 1000 times higher than the initial engraftment level in vivo [20]. After treatment, patients achieved complete remission (CR) [20]. Further, it has been recently reported that both patients sustained remission for more than ten years after the therapy and that CAR T cells are still detectable in their blood [21]. These long-persisting CAR T cells are CD4+ cells with cytotoxic capabilities, but also an expanded population of gamma delta CAR T cells has been found in one patient concomitant with CD8+ CAR T cells during the initial response phase [21].
So far, over 100 CLL patients have been treated with anti-CD19 CAR-T cells (Table 1). The majority of studies included relapsed patients or patients refractory to conventional therapy regimens, except for one which enrolled patients with only a partial response (PR) to the first line therapy [22]. Overall response rate (ORR; sum of CR and PR) varies between studies. Brentjens et al. suggested that chemotherapy that induced the depletion of lymphocytes prior to CAR T infusion enhanced the efficacy of CAR T cells [23]. In line with this are the results of the studies reporting the lowest ORR in patients without lymphodepletion therapy prior to CAR T infusion [23,24,25,26]. It is considered that lymphodepletion chemotherapy reduced tumor mass but also the number of regulatory cells, which may attenuate the antitumor activity of the infused CAR T cells.
Table 1. CAR T cells trials in CLL. Abbreviation: ORR—overall response rate, CR—complete response, CLL—chronic lymphocytic leukemia.
| Study (Reff. Number) | Number of CLL Patients | Target Antigen | Costimulatory Domain | ORR (%) | CR (%) |
|---|---|---|---|---|---|
| CAR-T | |||||
| [23] | 8 | CD19 | CD28 | 0 | 0 |
| [27] | 3 | CD19 | 4–1BB | 100 | 67 |
| [28] | 4 | CD19 | CD28 | 75 | 25 |
| [24] | 4 | CD19 | CD28 | 25 | 0 |
| [29] | 5 | CD19 | CD28 | 100 | 60 |
| [30] | 14 | CD19 | 4–1BB | 57 | 29 |
| [25] | 5 | CD19 | CD28 | 40 | 20 |
| [26] | 2 | IgKappa | CD28 | 0 | 0 |
| [31] | 32 | CD19 | 4–1BB | 44 | 28 |
| [13] | 24 | CD19 | 4–1BB | 71 | 17 |
| [22] | 8 | CD19 | CD28 | 75 | 25 |
| [32] | 19 | CD19 | 4–1BB | 53 | 53 |
| [33] | 10 | CD19 | 4–1BB | 60 | 40 |
| [34] | 19 | CD19 | 4–1BB | 79 | 21 |
| [35] | 3 | CD19 | 4–1BB | 100 | 67 |
Another factor that could influence response to therapy is a number of CLL T applied to patients. One recent study has shown that a higher dose of anti-CD19 CAR T cells (5.0 × 108 vs. 5.0 × 107) produces higher rates of ORR (55% vs. 31%) and CR (36% vs. 8%) [31].
This entry is adapted from the peer-reviewed paper 10.3390/curroncol29050293
This entry is offline, you can click here to edit this entry!
