Fruits are an important source of vitamins, minerals and nutrients in the human diet. They also contain several compounds of nutraceutical importance that have significant antioxidant and anti-inflammatory roles. Cherries contain high concentrations of bioactive compounds and minerals, including calcium, phosphorous, potassium and magnesium, and it is, therefore, unsurprising that cherry consumption has a positive impact on health. The sweet cherry fruit is a drupe—an indehiscent fruit of 1–2 cm in diameter (in some cultivars the diameter can be larger) that has an attractive appearance due to its color (bright red to dark purple depending on the cultivar) and desirable, intense flavor.
- tree fruit
- fruit ripening
- rootstock
- Prunus avium
1. Introduction
2. Cherry from Flower to Fruit
2.1. Cherry Flower Pollination
2.2. The ABCDE and the Floral Quartet Models
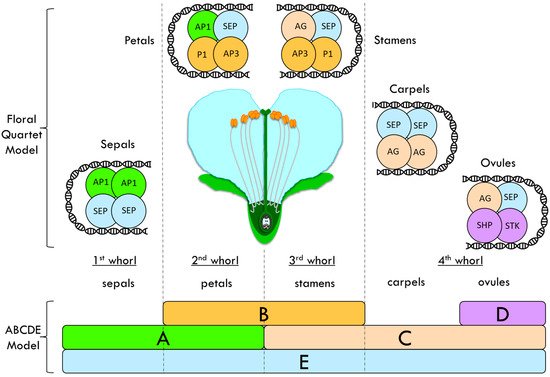
- MADS (M) domain: a DNA-binding domain, but it is also involved in dimerization and in nuclear localization. It is the most conserved domain among the MADS-box transcription factors [36].
- Intervening (I) domain: takes part in the selective formation of DNA-binding dimers. It shows only limited conservation [37].
- Keratin-like (K) domain: an essential element for dimerization and multimeric complex formation. This domain has a particular structural organization (amphipathic helices) in which the hydrophobic and charged residues are conserved and regularly spaced [38][39].
- C-terminal (C) domain: is somewhat variable. In some cases, it takes part in the transcriptional activation of the target genes or in the formation of multimeric complexes [37].
3. Cherry Fruit Development
3.1. Cherry Fruit
| Ripen | Days Post-Burlat * | Days Post Bing ** | Variety | Reported Qualities and Region of Origin |
|---|---|---|---|---|
| Burlat | 0 | NR | Burlat | The most widespread and cultivated cherry among existing varieties. It has good flavor, high resistance to cracking compared to most varieties, and exceeds in its time. |
| Early | 0–11 | NR | Merchant | Self-sterile, mature 7 days post-Burlat. Producing good yields of large, dark red fruit in early summer. Self-sterile, mature 9 days after Burlat. |
| NR | Carmen | Developed and cultivated in Hungary. Fruit have large size and good flavor. Spreading in Germany due to its high yields. | ||
| Mid | 12–19 | NR | Grace Star | Recent Italian variety. Self-fertile with maturation occurring 12 days post-Burlat. Large fruit, firm texture, excellent flavor qualities, and highly valued by consumers. |
| −5 | CristalinaTm | Self-sterile cherry of Canadian origin. Mature 12 days post-Burlat. High productivity, under conditions of moderate load. Fruit have good firmness and very good flavor and is moderately sensitive to cracking. | ||
| 0 | Stella | One of the first self-fertile varieties (Canadian origin). Ripens 19 days post-Burlat. Large fruit, blood-red hue, good resistance to cracking, sensitive to cold. Highly sought-after. Considered a cultivar of great importance. | ||
| Bing | Bing | Most traditional and representative cherry of America. Ripening occurs 19 days post-Burlat. Fruit are dark red, large, firm, and highly valued for their excellent flavor and is the preeminent fresh-market cherry. | ||
| 0 | Rainer | Bicolor cherry from the United States. The variety is self-sterile and ripens 19 days post-Burlat. Variety is appreciated by the industry owing to the large size and good flavor of the fruit. | ||
| Late | 20–27 | NR | Kordia | Originating in the Czech Republic with good resistance to cracking. Very popular in Germany. Ripens 24 days post-Burlat. Known for large, firm fruit and good flavor. Highly incompatible and setting virtually no fruit in the absence of cross-pollination. |
| 3 | SonataTm | Self-fertile Canadian variety with good productivity. Ripens 22 days post-Burlat. Fruit have a good taste and are large and firm in size. Unfortunately, it is highly susceptible to cracking. | ||
| 5–7 | Lapins | Highly productive self-fertile from the United States. Currently, most planted cherry in the world. Good flavor with cracking resistance. Variety is appreciated by farmers. Ripens 24 days post-Burlat (see SkeenaTm). | ||
| 7–10 | BentonTm | Another self-fertile cherry tree that ripens mid-season and has been reputed to surpass Bing cherries. | ||
| 10–14 | SkeenaTm | Characterized as an improved Lapins. Similar characteristics, but lower productivity, which helps to produce cherries of greater caliber and quality. In the United States, replacing Lapins. Mature 25 days post-Burlat. | ||
| 11–13 | SweetheartTm | Late maturation with large fruit. Prolific fruiters with a dark red, medium to large cherries. Pruning is required to keep trees productive. | ||
| Extra Late | More than 28 | NR | Regina | Self-sterile variety of German origin. Low productivity with maturation occurring 31 days post-Burlat. Large sized fruit, good taste and cultivated due to its high resistance to cracking. Highly incompatible and setting virtually no fruit in the absence of cross-pollination. |
| NR | Ambrunes | Spanish Cherry is traditionally grown in Cáceres. Firm fruit with very good flavor. High resistance to cracking. Ripens 31 days post-Burlat. |
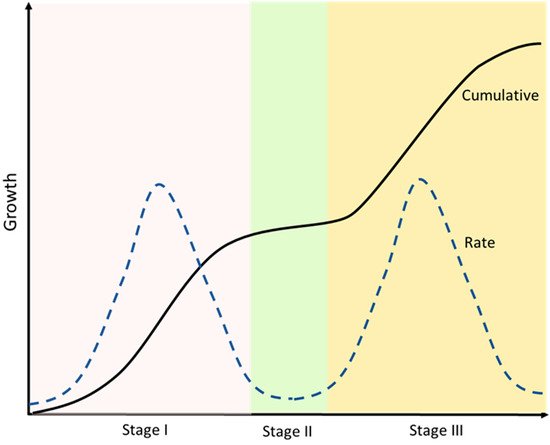
3.2. Physiological Changes during Cherry Fruit Development
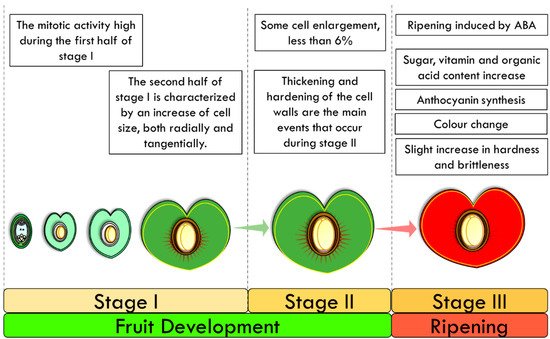
- The hypodermal layer of collenchyma;
- The peripheral layer of thin-walled parenchyma, extending to a line just inside the ring of vascular bundles;
- The layer of radially elongated cells, extending from this line nearly to the stone;
- The thin layer of small cells adjacent to the stone.
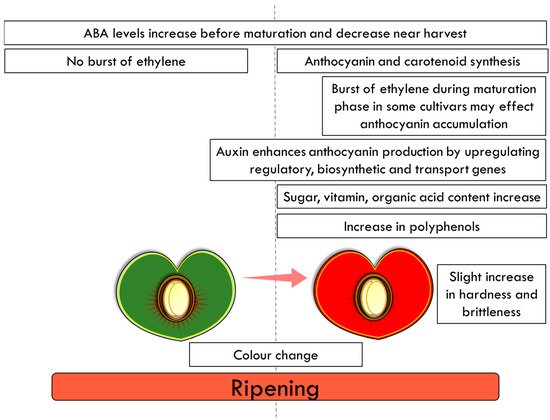
3.3. Final Ripening Stages of Mature Cherry
This entry is adapted from the peer-reviewed paper 10.3390/plants11121531
References
- Faust, M.; Surányi, D. Origin and dissemination of cherry. Hortic. Rev. 1997, 19, 263–317.
- Guajardo, V.; Solís, S.; Sagredo, B.; Gainza, F.; Muñoz, C.; Gasic, K.; Hinrichsen, P. Construction of high density sweet cherry (Prunus avium L.) linkage maps using microsatellite markers and SNPs detected by genotyping-by-sequencing (GBS). PLoS ONE 2015, 10, e0127750.
- Koepke, T.; Schaeffer, S.; Krishnan, V.; Jiwan, D.; Harper, A.; Whiting, M.; Oraguzie, N.; Dhingra, A. Rapid gene-based SNP and haplotype marker development in non-model eukaryotes using 3′UTR sequencing. BMC Genom. 2012, 13, 18.
- Tan, Q.; Li, S.; Zhang, Y.; Chen, M.; Wen, B.; Jiang, S.; Chen, X.; Fu, X.; Li, D.; Wu, H.; et al. Chromosome-level genome assemblies of five Prunus species and genome-wide association studies for key agronomic traits in peach. Hortic. Res. 2021, 8, 213.
- Jung, S.; Staton, M.; Lee, T.; Blenda, A.; Svancara, R.; Abbott, A.; Main, D. GDR (Genome Database for Rosaceae): Integrated web-database for Rosaceae genomics and genetics data. Nucleic Acids Res. 2008, 36, D1034–D1040.
- Scott, R.J.; Spielman, M.; Dickinson, H.G. Stamen structure and function. Plant Cell 2004, 16 (Suppl. 1), S46–S60.
- Abrol, D.P. Stone Fruits. In Pollination Biology, Vol. 1: Pests and Pollinators of Fruit Crops; Abrol, D.P., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 143–175.
- Srivastava, R.C.; Singh, I. Floral biology, fruit-set, fruit-drop and physicochemical characters of sweet-cherry (Prunus avium L.). Indian J. Agric. Sci. 1970, 40, 400–420.
- Kron, P.; Husband, B.C. The effects of pollen diversity on plant reproduction: Insights from apple. Sex. Plant Reprod. 2006, 19, 125–131.
- Ughini, V.; Roversi, A. Investigations on sweet cherry effective pollination period. Acta Hortic. 1996, 410, 423–426.
- Stösser, R.; Anvari, S.F. Pollen tube growth and fruit set as influenced by senescence of stigma, style and ovules. Acta Hortic. 1983, 139, 13–22.
- Roversi, A.; Ughini, V. How long should the period for a successful pollination of sweet cherry be? Acta Hortic. 1998, 468, 615–620.
- Thompson, M. Flowering, Pollination and Fruit Set. In Cherries: Crop Physiology, Production and Uses; Webster, A.D., Looney, N., Eds.; CAB International: Oxon, UK, 1996; pp. 223–241.
- Godini, A.; Palasciano, M.; Cozzi, G.; Petruzzi, G. Role of self-pollination and horticultural importance of self-compatibility in cherry. Acta Hortic. 1998, 468, 567–574.
- Hauck, N.R.; Yamane, H.; Tao, R.; Iezzoni, A.F. Self-compatibility and incompatibility in tetraploid sour cherry (Prunus cerasus L.). Sex. Plant Reprod. 2002, 15, 39–46.
- Dirlevander, E.; Claverie, J.; Wünsch, A.; Iezzoni, A.F. Cherry. In Genome Mapping and Molecular Breeding in Plants; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 4, pp. 103–118.
- Crane, M.B.; Brown, A.G. Incompatibility and sterility in the sweet cherry, Prunus avium L. J. Pomol. Hortic. Sci. 1938, 15, 116.
- Crane, M.B.; Lawrence, W.J.C. Genetical and cytological aspects of incompatibility and sterility in cultivated fruits. J. Pomol. Hortic. Sci. 1928, 7, 276–301.
- Sebolt, A.M.; Iezzoni, A.F. Utilization of the S-locus as a genetic marker in cherry to differentiate among different pollen donors. HortScience 2009, 44, 1542–1546.
- Wiersma, P.A.; Wu, Z.; Zhou, L.; Hampson, C.; Kappel, F. Identification of new self-incompatibility alleles in sweet cherry (Prunus avium L.) and clarification of incompatibility groups by PCR and sequencing analysis. Theor. Appl. Genet. 2001, 102, 700–708.
- Lech, W.; Małodobry, M.; Dziedzic, E.; Bieniasz, M.; Doniec, S. Biology of sweet cherry flowering. J. Fruit Ornam. Plant Res. 2008, 16, 189–199.
- Akšić, M.F.; Čolić, S.; Meland, M.; Natić, M. Sugar and polyphenolic diversity in floral nectar of cherry. In Co-Evolution of Secondary Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 755–773.
- Eeraerts, M.; Borremans, L.; Smagghe, G.; Meeus, I. A growers’ perspective on crop pollination and measures to manage the pollination service of wild pollinators in sweet cherry cultivation. Insects 2020, 11, 372.
- Holzschuh, A.; Dudenhöffer, J.-H.; Tscharntke, T. Landscapes with wild bee habitats enhance pollination, fruit set and yield of sweet cherry. Biol. Conserv. 2012, 153, 101–107.
- Eeraerts, M.; Smagghe, G.; Meeus, I. Pollinator diversity, floral resources and semi-natural habitat, instead of honey bees and intensive agriculture, enhance pollination service to sweet cherry. Agric. Ecosyst. Environ. 2019, 284, 106586.
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Effect of temperature on pollen tube kinetics and dynamics in sweet cherry, Prunus avium (Rosaceae). Am. J. Bot. 2004, 91, 558–564.
- Beppu, K.; Komatsu, N.; Yamane, H.; Yaegaki, H.; Yamaguchi, M.; Tao, R.; Kataoka, I. Se-haplotype confers self-compatibility in Japanese plum (Prunus salicina Lindl.). J. Hortic. Sci. Biotechnol. 2005, 80, 760–764.
- Beppu, K.; Kataoka, I. High temperature rather than drought stress is responsible for the occurrence of double pistils in ‘Satohnishiki’ sweet cherry. Sci. Hortic. 1999, 81, 125–134.
- Eaton, G.W. A study of the megagametophyte in P. avium and its relation to fruit setting. Can. J. Plant Sci. 1959, 39, 466–476.
- Bowman, J.L.; Smyth, D.R.; Meyerowitz, E.M. Genetic interactions among floral homeotic genes of Arabidopsis. Development 1991, 112, 1–20.
- Coen, E.S.; Meyerowitz, E.M. The war of the whorls: Genetic interactions controlling flower development. Nat. Commun. 1991, 353, 31–37.
- Theißen, G. Development of floral organ identity: Stories from the MADS house. Curr. Opin. Plant Biol. 2001, 4, 75–85.
- Goto, K.; Kyozuka, J.; Bowman, J.L. Turning floral organs into leaves, leaves into floral organs. Curr. Opin. Genet. Dev. 2001, 11, 449–456.
- Theißen, G.; Melzer, R.; Rümpler, F. MADS-domain transcription factors and the floral quartet model of flower development: Linking plant development and evolution. Development 2016, 143, 3259–3271.
- Theißen, G.; Saedler, H. Floral quartets. Nat. Commun. 2001, 409, 469–471.
- Gramzow, L.; Theissen, G. A hitchhikers guide to the MADS world of plants. Genome Biol. 2010, 11, 214.
- Kaufmann, K.; Melzer, R.; Theissen, G. MIKC-type MADS-domain proteins: Structural modularity, protein interactions and network evolution in land plants. Gene 2005, 347, 183–198.
- Puranik, S.; Acajjaoui, S.; Conn, S.; Costa, L.; Conn, V.; Vial, A.; Marcellin, R.; Melzer, R.; Brown, E.; Hart, D.; et al. Structural basis for the oligomerization of the MADS domain transcription factor SEPALLATA3 in Arabidopsis. Plant Cell 2014, 26, 3603–3615.
- Yang, Y.; Fanning, L.; Jack, T. The K domain mediates heterodimerization of the Arabidopsis floral organ identity proteins, APETALA3 and PISTILLATA. Plant J. 2003, 33, 47–59.
- Theißen, G.; Gramzow, L. Chapter 8—Structure and evolution of plant MADS domain transcription factors. In Plant Transcription Factors: Evolutionary, Structural and Functional Aspects; Gonzalez, D.H., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 127–138.
- Varoquaux, F.; Blanvillain, R.; Delseny, M.; Gallois, P. Less is better: New approaches for seedless fruit production. Trends Biotechnol. 2000, 18, 233–242.
- Dorcey, E.; Urbez, C.; Blázquez, M.A.; Carbonell, J.; Perez-Amador, M.A. Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J. 2009, 58, 318–332.
- Vriezen, W.H.; Feron, R.; Maretto, F.; Keijman, J.; Mariani, C. Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytol. 2008, 177, 60–76.
- Wang, H.; Schauer, N.; Usadel, B.; Frasse, P.; Zouine, M.; Hernould, M.; Latché, A.; Pech, J.C.; Fernie, A.R.; Bouzayen, M. Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell 2009, 21, 1428–1452.
- Tukey, H.B. Growth of the embryo, seed, and pericarp of the sour cherry (Prunus cerasus) in relation to season of fruit ripening. Proc. Am. Soc. Hortic. Sci. 1934, 31, 125–144.
- Lilleland, O. Growth study of the plum fruit-I. The growth and changes in chemical composition of the climax plum. In Proceeding of the American Society for Horticultural Science; American Society for Horticultural Science: Alexandria, VA, USA, 1933; Volume 30, pp. 203–208.
- Lilleland, O. Growth study of the peach fruit. In Proceeding of the American Society for Horticultural Science; American Society for Horticultural Science: Alexandria, VA, USA, 1935; Volume 33, pp. 269–279.
- Coombe, B.G. The development of fleshy fruits. Annu. Rev. Plant Physiol. 1976, 27, 207–228.
- Bollard, E.G. The physiology and nutrition of developing fruit. In The Biochemistry of Fruit and Their Products; Hulme, A.C., Rhodes, M.J., Eds.; FAO of the United Nations: Rome, Italy, 1970; Volume 1.
- Lilleland, O.; Newsome, L. A growth study of the cherry fruit. Proc. Am. Soc. Hortic. Sci. 1934, 32, 291–299.
- Chalmers, D.J.; Ende, B.V.D. A reappraisal of the growth and development of peach fruit. Funct. Plant Biol. 1975, 2, 623–634.
- Gibeaut, D.M.; Whiting, M.D.; Einhorn, T. Time indices of multiphasic development in genotypes of sweet cherry are similar from dormancy to cessation of pit growth. Ann. Bot. 2017, 119, 465–475.
- Tukey, H.B.; Young, J.O. Histological study of the developing fruit of the sour cherry. Bot. Gaz. 1939, 100, 723–749.
- Farmer, J.B. Contributions to the morphology and physiology of pulpy fruits. Ann. Bot. 1889, 3, 393–414.
- Winton, A.L. The anatomy of edible berries. Am. J. Pharm. (1835–1907) 1904, 439, 533.
- Kapoor, L.; Simkin, A.J.; George Priya Doss, C.; Siva, R. Fruit ripening: Dynamics and integrated analysis of carotenoids and anthocyanins. BMC Plant Biol. 2022, 22, 27.
- Fukano, Y.; Tachiki, Y. Evolutionary ecology of climacteric and non-climacteric fruits. Biol. Lett. 2021, 17, 20210352.
- Paul, V.; Pandey, R.; Srivastava, G.C. The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene-An overview. J. Food Sci. Technol. 2012, 49, 1–21.
- Klee, H.J.; Giovannoni, J.J. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011, 45, 41–59.
- Giovannoni, J.J. Genetic regulation of fruit development and ripening. Plant Cell 2004, 16 (Suppl. 1), S170–S180.
- Setha, S.; Kondo, S.; Hirai, N.; Ohigashi, H. Quantification of ABA and its metabolites in sweet cherries usingdeuterium-labeled internal standards. Plant Growth Regul. 2005, 45, 183–188.
- Gong, Y.; Fan, X.; Mattheis, J.P. Responses of ‘Bing’ and ‘Rainier’ sweet cherries to ethylene and 1-methylcyclopropene. J. Am. Soc. Hortic. Sci. JASHS 2002, 127, 831–835.
- Li, S.; Andrews, P.K.; Patterson, M.E. Effects of ethephon on the respiration and ethylene evolution of sweet cherry (Prunus avium L.) fruit at different development stages. Postharvest Biol. Technol. 1994, 4, 235–243.
- Kondo, S.; Gemma, H. Relationship between Abscisic Acid (ABA) content and maturation of the sweet cherry. J. JPN Soc. Hortic. Sci. 1993, 62, 63–68.
- Hartmann, C. Biochemical changes in harvested cherries. Postharvest Biol. Technol. 1992, 1, 231–240.
- Kondo, S.; Inoue, K. Abscisic acid (ABA) and 1-aminocyclopropane-l-carboxylic acid (ACC) content during growth of ‘Satohnishiki’ cherry fruit, and the effect of ABA and ethephon application on fruit quality. J. Hortic. Sci. 1997, 72, 221–227.
- Ishiguro, T.; Yamaguchi, M.; Nishimura, K.; Satoh, I. Changes of fruit characteristics and respiration in sweet cherry (Prunus avium L.) during ripening. J. JPN Soc. Hortic. Sci. 1993, 62, 146–147.
- Hartmann, C. Ethylene and ripening of a non-climacteric fruit: The cherry. Acta Hortic. 1989, 258, 89–96.
- Ren, J.; Chen, P.; Dai, S.J.; Li, P.; Li, Q.; Ji, K.; Wang, Y.P.; Leng, P. Role of abscisic acid and ethylene in sweet cherry fruit maturation: Molecular aspects. N. Z. J. Crop Hortic. Sci. 2011, 39, 161–174.
- Castellarin, S.D.; Gambetta, G.A.; Wada, H.; Krasnow, M.N.; Cramer, G.R.; Peterlunger, E.; Shackel, K.A.; Matthews, M.A. Characterization of major ripening events during softening in grape: Turgor, sugar accumulation, abscisic acid metabolism, colour development, and their relationship with growth. J. Exp. Bot. 2015, 67, 709–722.
- Zhang, M.; Yuan, B.; Leng, P. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J. Exp. Bot. 2009, 60, 1579–1588.
- Coombe, B.G.; Hale, C.R. The hormone content of ripening grape berries and the effects of growth substance treatments. Plant Physiol. 1973, 51, 629–634.
- Luo, H.; Dai, S.; Ren, J.; Zhang, C.; Ding, Y.; Li, Z.; Sun, Y.; Ji, K.; Wang, Y.; Li, Q.; et al. The role of ABA in the maturation and postharvest life of a monclimacteric sweet cherry fruit. J. Plant Growth Regul. 2014, 33, 373–383.
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493.
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins. Phytother. Res. 2016, 30, 1265–1286.
- Clayton-Cuch, D.; Yu, L.; Shirley, N.; Bradley, D.; Bulone, V.; Böttcher, C. Auxin treatment enhances anthocyanin production in the non-climacteric sweet cherry (Prunus avium L.). Int. J. Mol. Sci. 2021, 22, 10760.
- Kappel, F.; Fisher-Fleming, B.; Hogue, E. Fruit characteristics and sensory attributes of an ideal sweet cherry. HortScience 1996, 31, 443–446.
- Zheng, X.; Yue, C.; Gallardo, K.; McCracken, V.; Luby, J.; McFerson, J. What attributes are consumers looking for in sweet cherries? Evidence from choice experiments. Agric. Resour. Econ. Rev. 2016, 45, 124–142.
- Guyer, D.E.; Sinha, N.K.; Chang, T.S.; Cash, J.N. Physiochemical and sensory characteristics of selected Michigan sweet cherry (Prunus avium L.) cultivars. J. Food Qual. 1993, 16, 355–370.
- Cliff, M.A.; Dever, M.C.; Hall, J.W.; Giraud, B. Development and evaluation of multiple regression models for predicting sweet cherry liking. Food Res. Int. 1996, 28, 583–589.
- Lyngstad, L.; Sekse, L. Economic aspects of developing a high sweet cherry product in Norway. Acta Hortic. 1995, 379, 313–320.
- Sekse, L.; Lyngstad, L. Strategies for maintaining high quality in sweet cherries during harvesting, handling and marketing. Acta Hortic. 1996, 410, 351–355.
- Wermund, U.; Fearne, A. Key challenges facing the cherry supply chain in the UK. Acta Hortic. 2000, 536, 613–624.
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer acceptance of ‘Brooks’ and ‘Bing’ cherries is mainly dependent on fruit SSC and visual skin color. Postharvest Biol. Technol. 2003, 28, 159–167.
