1. Autophagy in Cell Survival and Cell Death
One such apoptosis-independent cell death mechanism relies on the over activation of autophagy, a cellular stress response that normally serves as a quality control mechanism. Different forms of autophagy have been described including macroautophagy (hereafter referred to as autophagy), microautophagy, and chaperone-mediated autophagy. The molecular basis of mammalian autophagy is depicted in Figure 1.
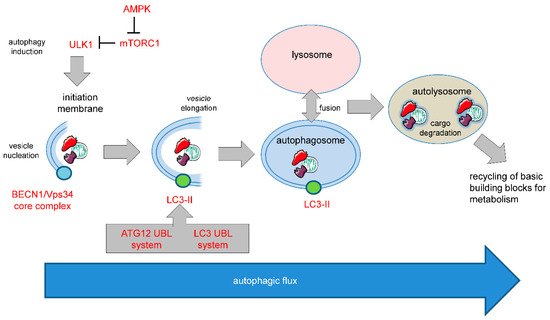
Figure 1. Molecular basis of mammalian autophagy. Autophagy is a multistep process involving several key ATG proteins and signaling complexes. It requires the formation of double-membrane-containing autophagosomes that sequester proteins, lipids, organelles or invasive microbes and fuse with lysosomes for digestion of content by acidic hydrolases. ULK1, a protein kinase serving as the central initiator of autophagy, is inhibited by the mTORC1 complex that contains mTOR. AMPK serves as a nutrient sensor and negative regulator of mTORC1. Autophagosome biogenesis starts with the formation of an initiation membrane that is derived either from the endoplasmatic reticulum (ER) or from several other cellular membrane sources. Vesicle nucleation is promoted by the BECN1/Vps34 core complex containing the lipid kinase Vps34. Vesicle elongation is regulated by the two ubiquitin-like conjugation systems (UBLs) ATG12-UBL and LC3-UBL that cooperate to catalyze the conjugation of phosphatidylethanolamine (PE) to LC3 and facilitate the conversion of cytosolic LC3-I into a membrane-associated LC3-II that is translocated to the autophagosomal membrane. Following vesicle closure, mature autophagosomes fuse with lysosomes to generate autolysosomes that digest the autophagosomal content by lysosomal proteases for cellular recycling [
5]. This figure was created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License;
https://smart.servier.com.
In general, autophagy is a pro-survival stress response, for example, autophagy will be activated under situations of nutrient deprivation to ensure supply of basic building blocks for metabolism and survival of the cells/organisms by recycling of non-essential cellular components. Autophagy also serves to remove damaged and potentially harmful organelles, thereby supporting cell survival. On the other hand, there is conclusive evidence that prolonged over activation of the autophagosomal/lysosomal pathway can lead to autophagic cell death (ACD, type II cell death). Of note, similar threshold effects on cell survival vs cell death are commonly observed in various stress responses like the endoplasmatic reticulum (ER) stress response and activation of p53 [
6]. Accordingly, ACD is often described as self-digestion beyond the point allowing cell survival [
3,
4,
6,
7,
8]. Hence, the net effect of autophagy on cell survival is highly dependent on its intensity and duration, but also on its particular context (
Figure 2).
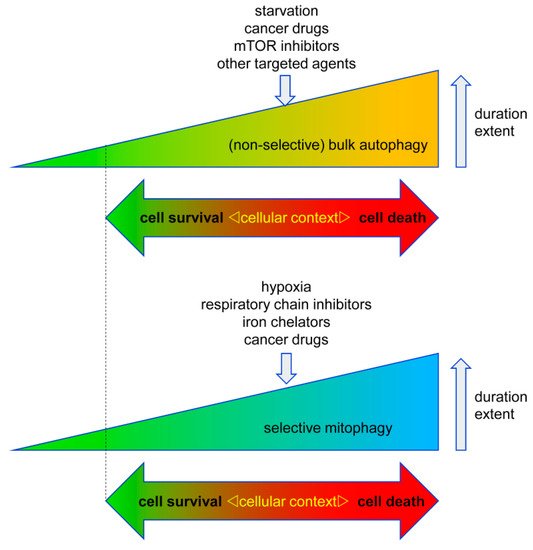
Figure 2. Context-dependent cell responses to bulk autophagy and selective mitophagy. Both autophagy and mitophagy can either promote or inhibit cell death in cancer cells. This response is highly dependent on the cell type, the trigger of auto-/mitophagy, its duration, and its extent. Accordingly, excessive autophagy can lead to cell death, however too little autophagy/mitophagy (marked by dotted line) can also be detrimental to the cells due to an impaired quality control/removal of harmful cellular material.
2. Autophagy in Tumorigenesis and Tumor Progression
Autophagy is not only involved in the responses to tumor therapy (see below), but also plays a key role in cancer development. This involvement of autophagy in tumorigenesis and tumor progression is very complex and multifaceted. It is hypothesized that in the early stages of tumorigenesis, autophagy exerts a tumor-suppressive function. This is based on the observation that an intact autophagy pathway correlates with decreased oxidative stress and increased genomic stability [
8], thereby ensuring the survival of healthy, non-transformed cells. On the other hand, it was recently proposed that autophagy may also support cancer progression by facilitating tumor cell survival and fitness under replication stress, a common feature of most malignancies [
21].
In line with the high context-dependency of autophagy, there is a shift from tumor-suppressing to tumor-promoting autophagy during the course of tumor progression. It is believed that autophagy can alleviate the stressful environmental conditions like hypoxia or nutrient deprivation often encountered in manifest, solid tumors [
28,
29], but also in non-malignant ischemic tissues [
30], rendering the tumors more stress-resistant. Using a
Drosophila model, Katheder et al. recently showed that not only tumor-intrinsic but also microenvironmental autophagy is capable of inducing tumor growth by providing the nutrients necessary for tumor growth [
31]. They could further demonstrate that this was achieved through elevated ROS levels due to mitochondrial damage in the tumor cells, which induced nutrient export from the microenvironment.
Since another key hallmark of cancer is chronic inflammation, it will also be of key importance to better understand the mutual interplay between autophagy and inflammation. There is evidence suggesting that autophagy can either suppress or promote inflammation in cancer. Likewise, inflammatory pathways can either suppress or induce autophagy in a context-dependent manner. A complex scenario is recently emerging that will aid future studies aimed at deciphering the exact role of autophagy in shaping the immune and inflammatory microenvironment of tumors [
32].
In addition to supporting tumor growth in general, autophagy has been demonstrated to regulate and/or maintain the cancer stem cell phenotype and treatment resistance in multiple studies, for example, in oral squamous cell carcinoma [
33] and endometrial cancer [
34].
3. Autophagy in Therapy Response
Next to its role in tumorigenesis and malignant progression, autophagy plays a key role in cancer therapy responses. Given the dual function of autophagy in cell survival vs cell death, inhibition, but also over activation of autophagy, carries potential relevance for therapy.
3.1. Pro-Survival Autophagy
Since autophagy appears to act mainly as a pro-survival stress response that is activated (at least to some degree) by most, if not all, conventional cancer drugs and by radiation, pro-survival autophagy is expected to hamper the effects of cancer therapy in most settings. Some examples of a therapy resistance-increasing effect of autophagy are listed below. The impact of pro-survival autophagy in cancer therapy was extensively covered elsewhere in our recent review [
35] where we also delineated the molecular mechanisms of autophagy regulation in response to therapy-related stress conditions in this context, and we refer the reader to this work for further details. Two central cellular players involved in many paradigms of pro-survival autophagy of cancer cells are mTOR and AMPK (
Figure 1) that are often involved in activation of autophagy as an unwanted side effect of different cancer drugs/treatments. For example, treatment with Taxol was shown to activate pro-survival autophagy caused by inhibition of mTOR in breast cancer cells [
36].
For pancreatic cancer, it has been shown that primary tumors and cell lines exhibit increased autophagy, while autophagy inhibition (genetic and pharmacological) results in increased reactive oxygen species (ROS) formation and DNA damage, while treatment of tumor-bearing mice with the autophagic flux-inhibitor chloroquine (CQ) improved overall survival [
40]. In another study Qiu et al. showed that autophagy induced by cisplatin protected ovarian cancer cells [
41], while DeVorkin et al. could, in fact, show that cancer cells of clear-cell ovarian cancer depend on autophagy for their survival [
42]. From a mechanistic perspective, it should, however, be noted that CQ is not a highly selective inhibitor of autophagy. A recent study demonstrated that CQ also has profound non-autophagic effects on cells, especially concerning disorganization of the Golgi and endo-lysosomal systems [
43], arguing for a more cautious interpretation of responses to CQ.
The approach of combining conventional or targeted therapy with autophagy inhibition (CQ, Hydroxy-CQ) is currently also investigated in several clinical studies in patients with various types of cancer, including glioblastoma. Accordingly, Jutten et al. showed recently that glioma cells expressing mutant EGFRvIII that is associated with poor prognosis [
44] and occurs in half of all glioblastoma patients [
45], are more sensitive to CQ treatment, and hence rely more strongly on autophagy for cell survival. Most importantly, using a retrospective analysis, this study also showed that patients with mutant EGFRvIII receiving CQ have the highest benefit of CQ-treated patients [
46]. Another recent, very promising study provided evidence that autophagy inhibition can be employed to overcome therapy resistance of brain tumor patients against BRAF inhibitor treatment [
47].
3.2. Pro-Death Autophagy
Given the fact that genetic and pharmacological abrogation of autophagy inhibits non-selective as well as selective types of autophagy, it is currently not well understood whether excessive pro-death bulk autophagy, i.e., non-selective autophagy, is the (solely) responsible type of autophagy for cell killing in most established paradigms of ACD, including ACD in lower organisms and ACD induced by cancer drugs. The following section lists several examples from the literature that lack evidence for a death-promoting contribution of selective autophagy pathways, such as mitophagy (see next paragraph).
Resveratrol, a polyphenolic compound found in red wine [
48], has been described to induce bona fide ACD in chronic myeloid leukemia [
15] and induces cell death in prostate [
49], ovarian [
16], and endometrial cancer cells [
50] that involves induction of autophagy, although the latter studies failed to provide complete evidence that the criteria required by the NCCD [
3] are fulfilled. A recent shRNA-based screen of A549 lung cancer cells analyzed potential regulators of resveratrol-induced ACD and identified glucosylceramidase beta (GBA1) as a potential mediator of ACD [
51]. ACD has also been observed in cells treated with Interferon-gamma (IFN-γ) which induced cell death that could be rescued after treatment with the autophagy-inhibitor 3-methyl-adenine (3MA) or knockdown of ATG5 [
17]. Based on the observations that cancer cells have a higher turnover rate of NAD+, this pathway was recently employed to target cancer cells by triggering ACD via inhibition of the NAD+-synthesizing enzyme Nampt using the inhibitor FK866 in myeloma [
52] or by inhibition of the nicotinamide phosphoribosyltransferase by APO866 in leukemia and lymphoma cells [
53]. Lima et al. used SK1-I, an inhibitor of sphingosine kinase 1 (SPHK1) and analog of sphingosine, in colon cancer cell lines and observed induction of autophagy and cell death which was dependent on BECN1 and ATG5 [
54], although in this study the discrimination between apoptosis and autophagy is not entirely clear, leaving some room for interpretation if the mode of death can be truly defined as ACD according to the NCCD criteria. Other groups showed that downregulation of the AKT1/mTOR-axis using the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) induced ACD in hepatocellular carcinoma (HCC) cell lines [
55]. Finally, the cholesterol metabolite dendrogenin A (DDA) induced lethal autophagy, reminiscent of ACD, in myeloma and acute myeloid leukemia in vitro and in vivo [
19].
Arsenic trioxide was shown to induce ACD and cell death in various tumor cell populations in multiple studies, including our own [
14,
56,
57,
58]. Considering that arsenic trioxide is already clinically used to treat acute promyelocytic leukemia (APL) [
59] and easily crosses the blood-brain-barrier [
60], this drug could be particularly interesting for hard-to-treat cancers, such as brain tumors (primary or metastases). In particular, it was shown that arsenic trioxide-induced ACD is mediated by the protein BNIP3 (BCL2 interacting protein 3) and BNIP3L (BCL2 interacting protein 3 like; also known as NIX) [
14], that were subsequently identified as mitophagy-receptors [
61]. These findings imply that arsenic trioxide triggers selective autophagy of mitochondria (mitophagy) in addition to non-selective bulk-autophagy, with possible implications for cell death activation. However, this proposition warrants future research.
This entry is adapted from the peer-reviewed paper 10.3390/biology8040082