Bone defects and complex fractures present significant challenges for orthopaedic surgeons. Current surgical procedures involve the reconstruction and mechanical stabilisation of complex fractures using metal hardware (i.e., wires, plates and screws). However, these procedures often result in poor healing. An injectable, biocompatible, biodegradable bone adhesive that could glue bone fragments back together would present a highly attractive solution.
- bone fractures
- bioadhesives
- bone repairing
- biomimetic adhesives
1. Introduction
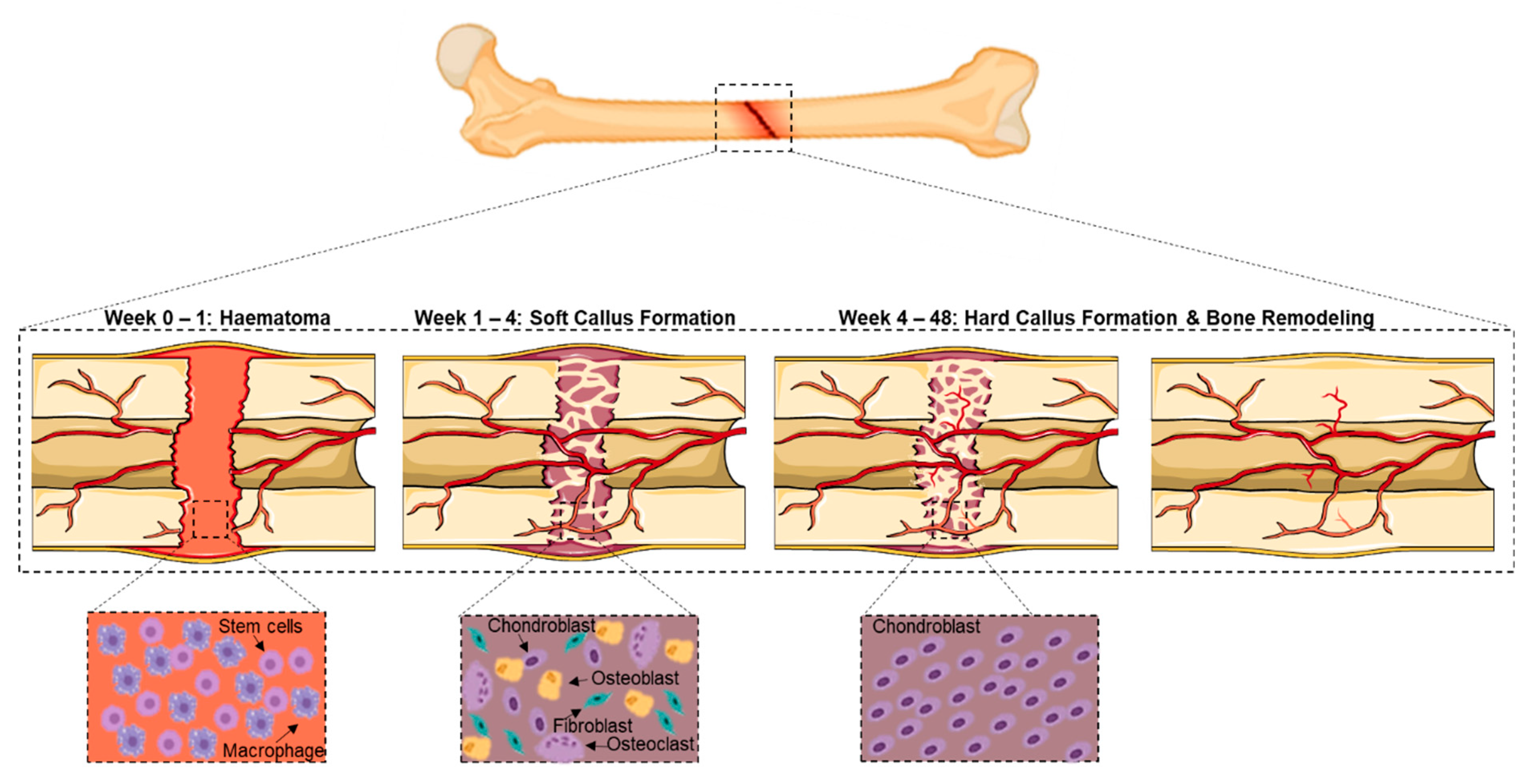
2. Complex Bone Fractures
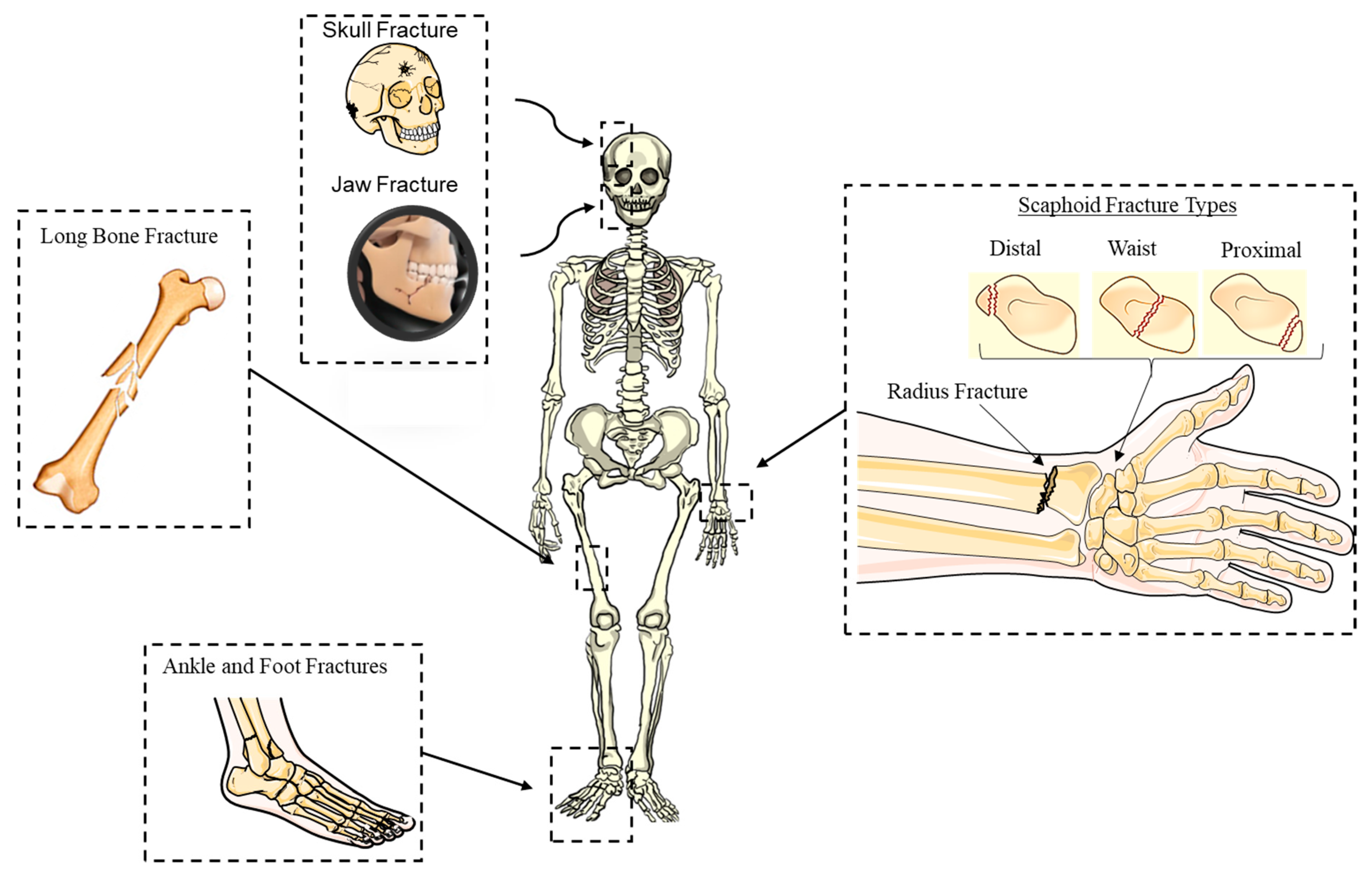
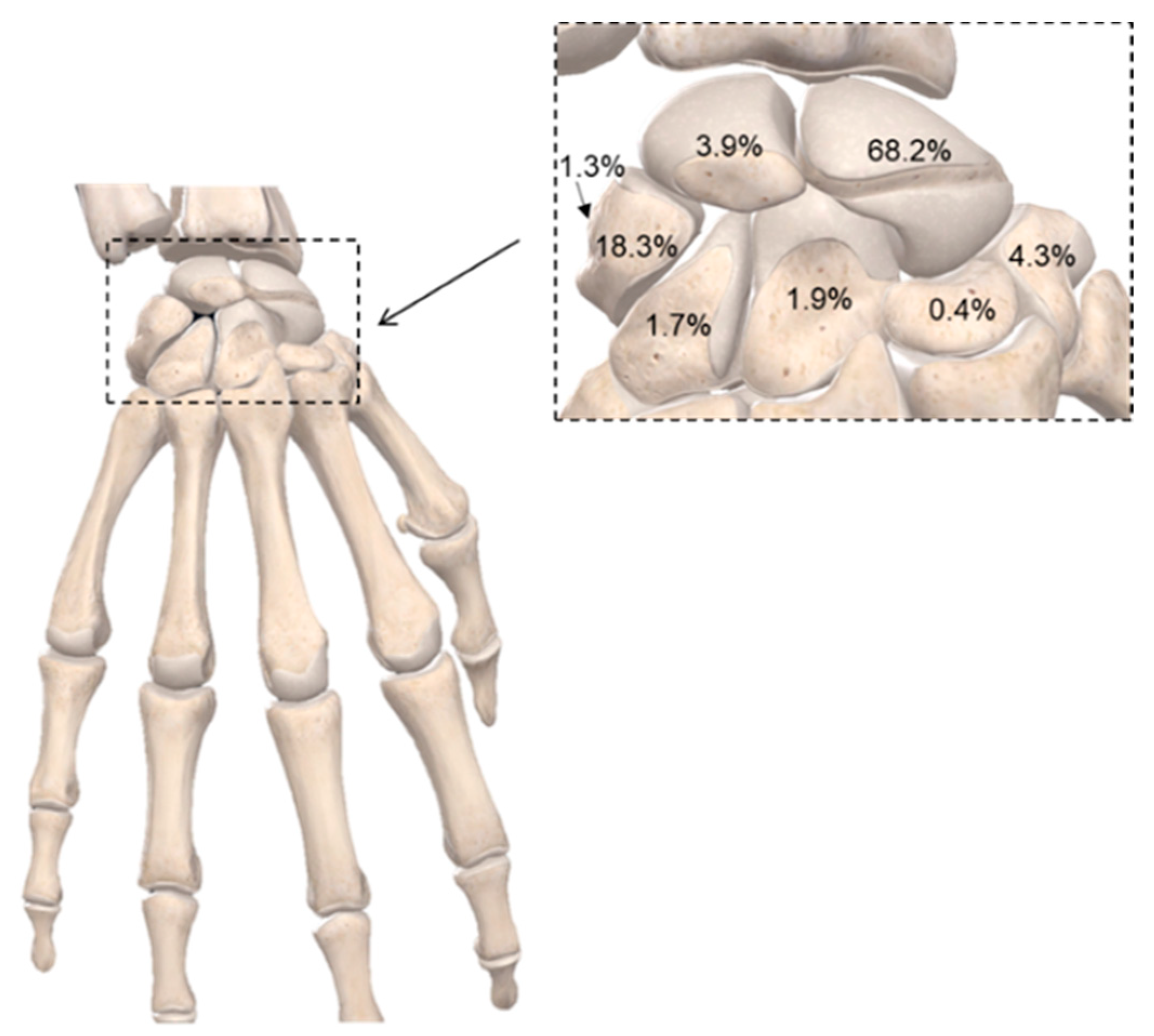
3. Current Surgical Approaches for Fracture Repair
4. Bioadhesives
4.1. Synthetic Bioadhesives
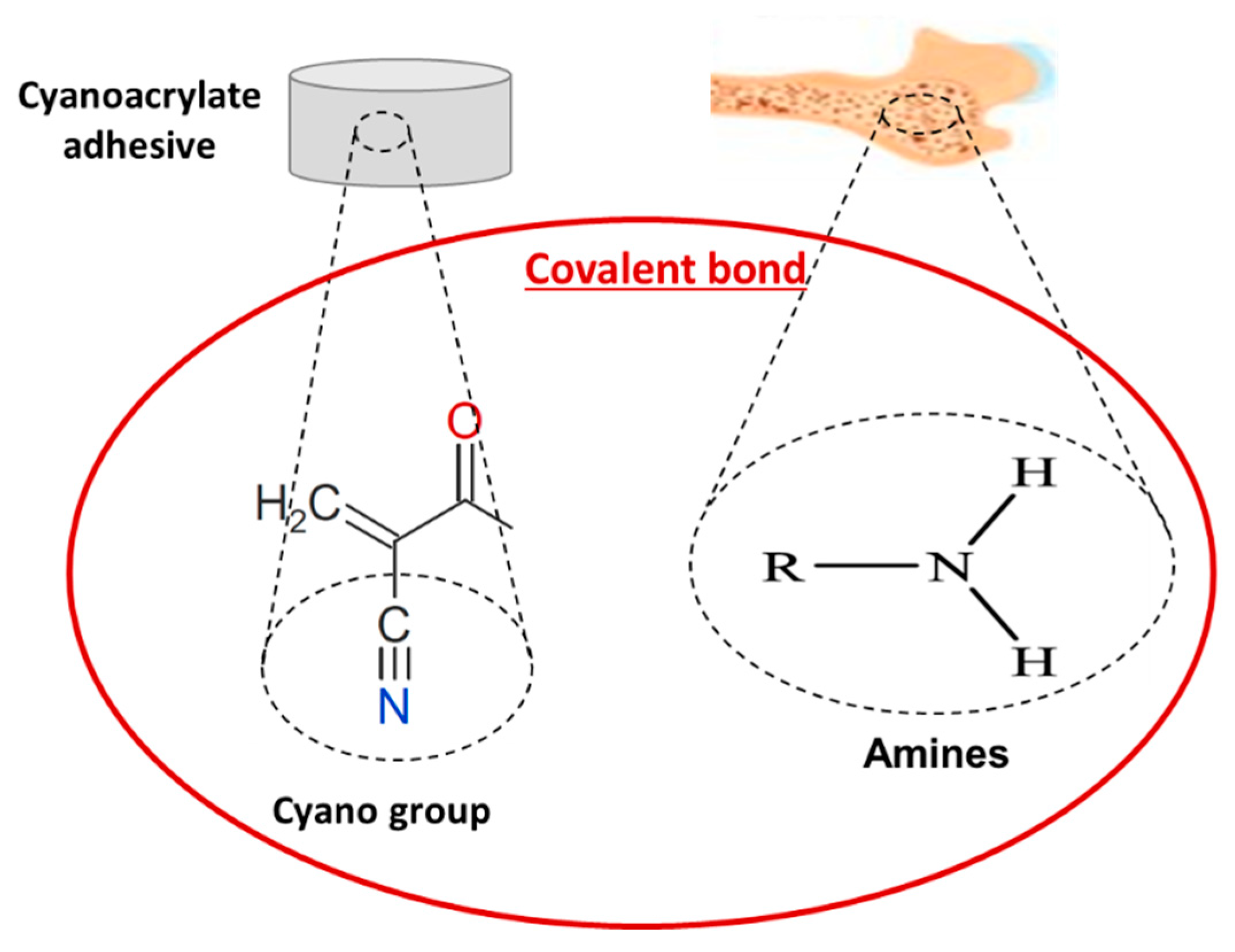
4.1.1. Cyanoacrylates
4.1.2. Polyurethanes
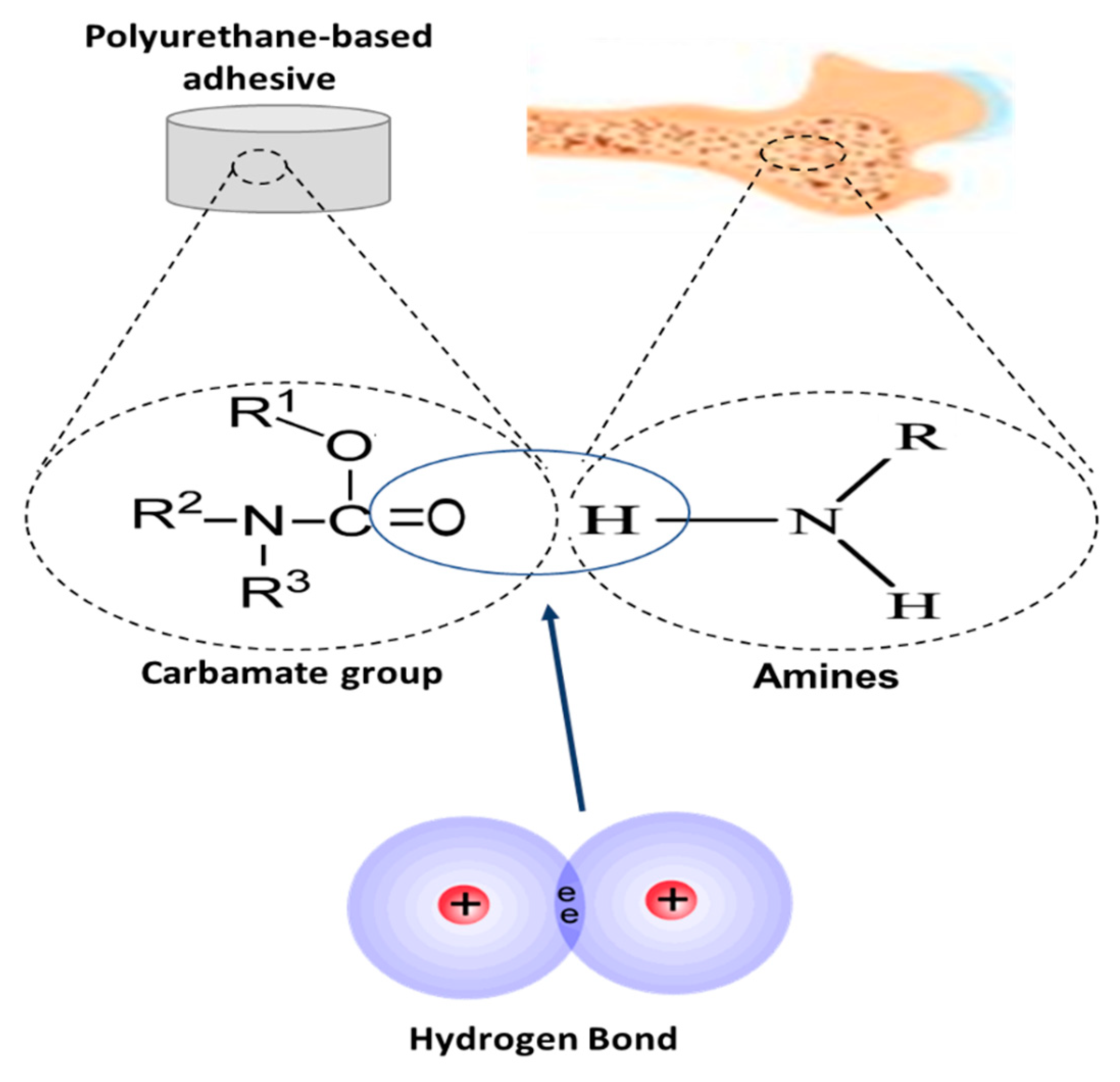
4.1.3. Polyesters
4.1.4. Poly-methyl Methacrylates (PMMA)
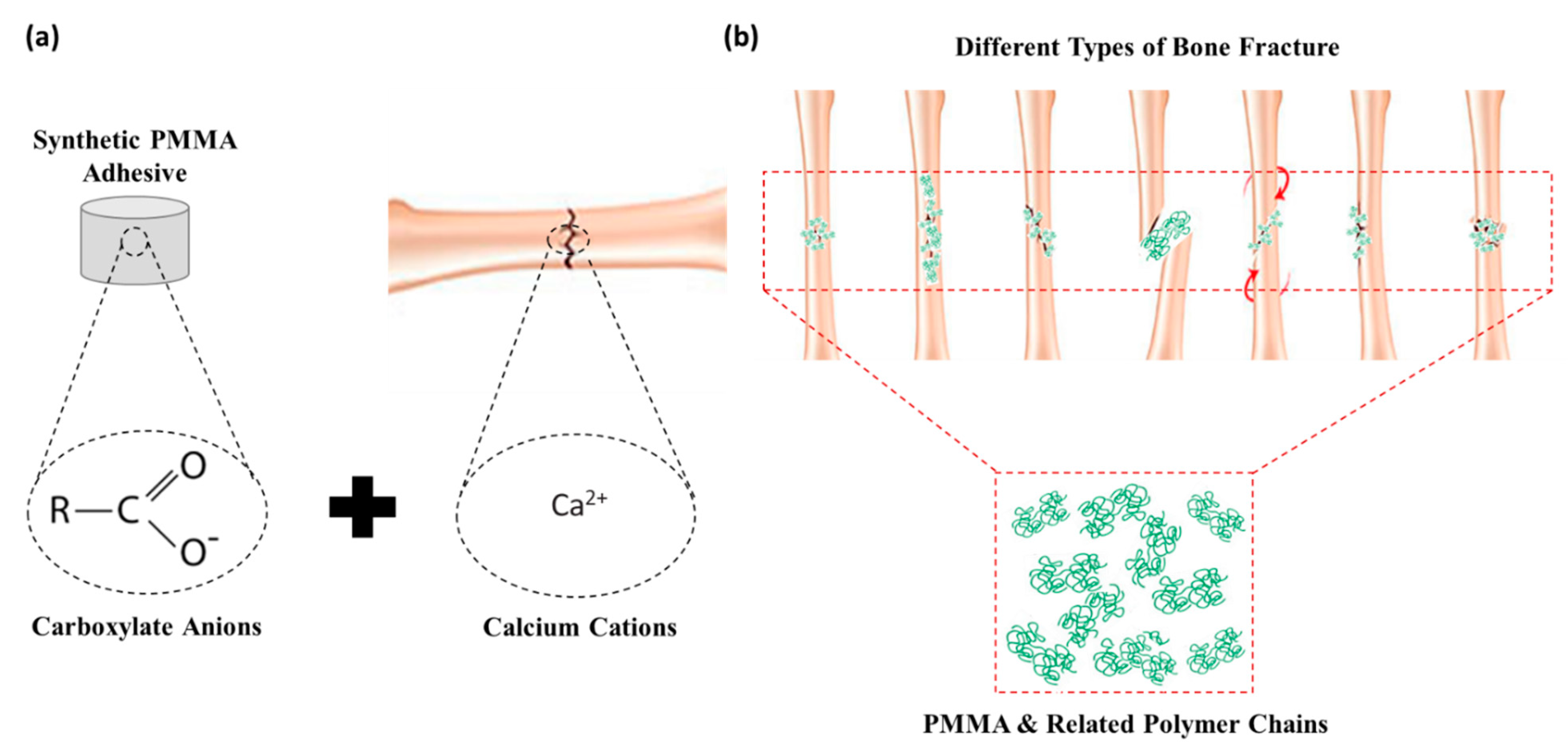
|
Scheme |
|||
|---|---|---|---|
|
Application |
Advantages |
Disadvantages |
|
|
Cyanoacrylates |
Craniofacial, osteochondral and trabecular fractures Bone formation and fragments fixation Enhancement or replacement of screws/plates |
Max adhesive strength of 9 MPa Enhanced tensile and shear bond in wet and dry environment Higher shear strength (1–2 MPa) than screws and plates |
Partial bone formation Less efficient than screws with low adhesive and mechanical properties Chronic inflammatory response and tissue necrosis Cytotoxicity to cells in vitro and dermatitis in vivo |
|
Bone formation and fragments fixation Bone to bone adhesion Closure of fractures |
High adhesive or/and cohesive strength Osteogenic, non-toxic and biocompatible Degradation in wet environment |
Bond failure between bone and adhesive Low biodegradability Infection Tissue necrosis |
|
|
Polyester |
Scaffold in bone regeneration Tissue adhesion |
Faster degradation in wet environment than polyurethane-based High mechanical & adhesion strength |
Mechanical stability during degradation Osteogenic capacities (osteoconduction and osteoinduction) Inflammation at the application site Low yield strength Significant cytotoxicity |
|
Poly-methyl methacrylate (PMMA) |
Bone fragment and implant fixation Adhesives in dentistry Bone formation |
Hydrophobic behaviour Increased bonding to wet bone Easy application Cytocompatibility |
Low adhesive strength Thermal necrosis of bone tissue Lack of biodegradability |
4.2. Naturally-Derived Bioadhesives
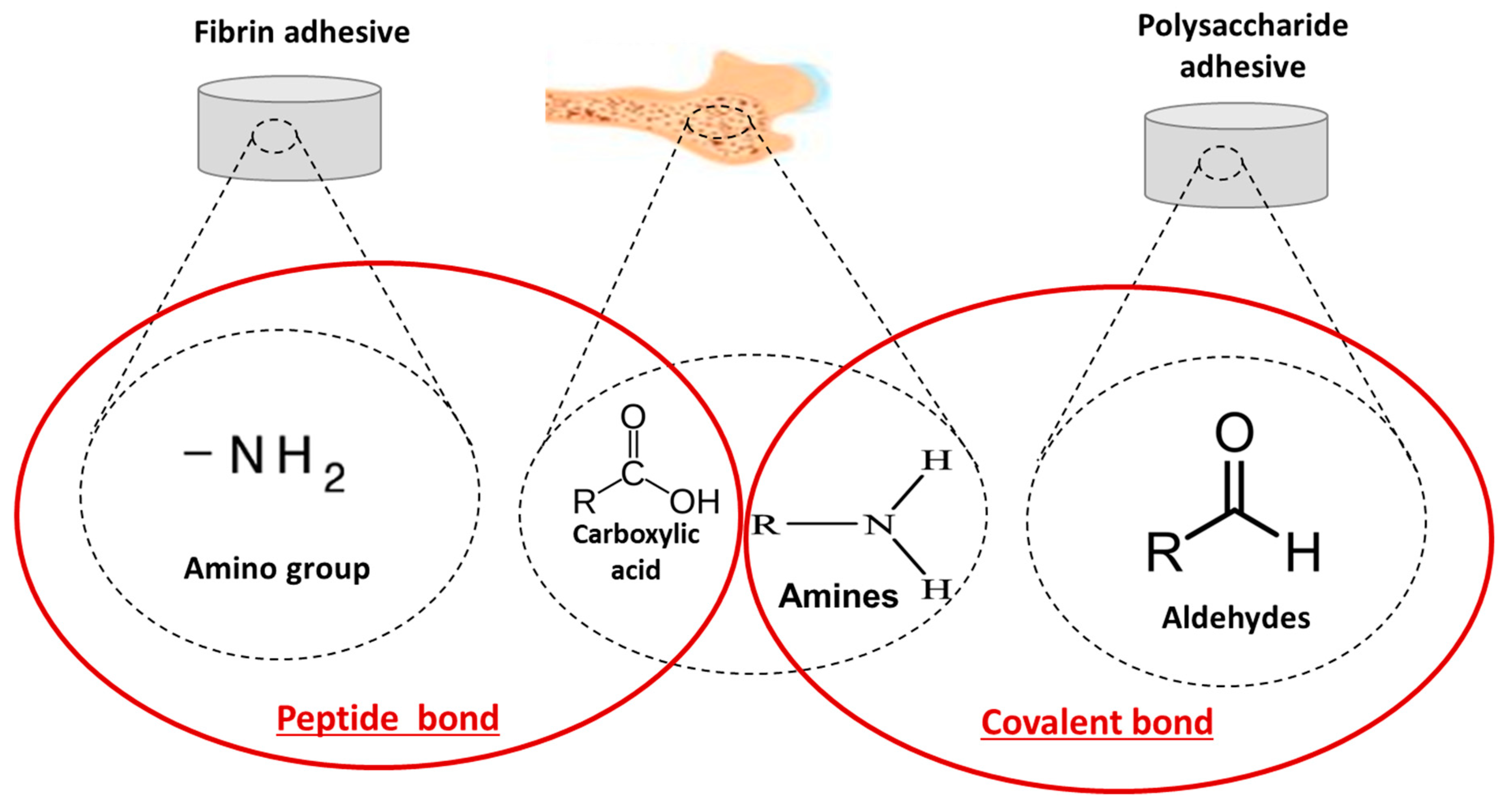
4.3. Biomimetic-Based Adhesives
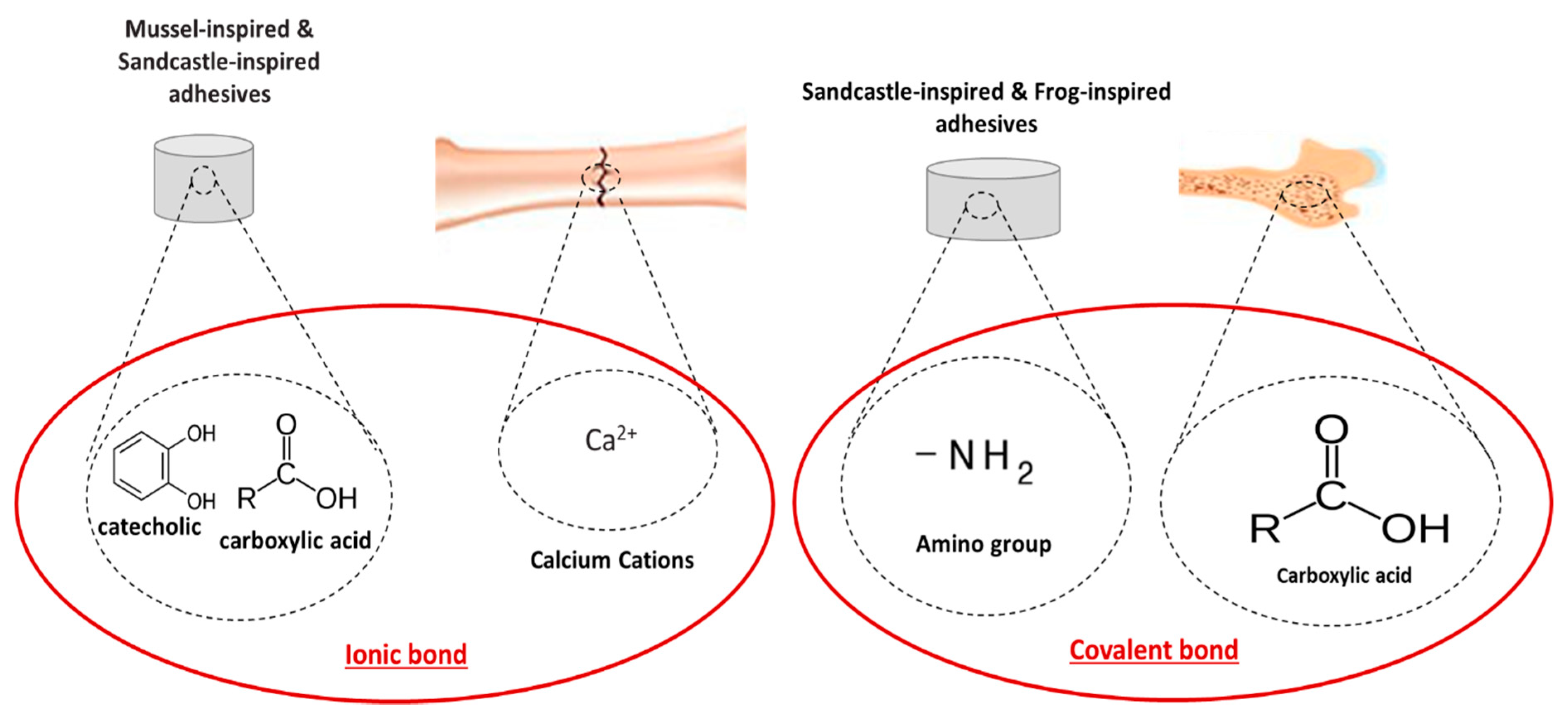
|
Biomimetic Adhesives |
||||
|---|---|---|---|---|
|
Description |
Application |
Advantages |
Disadvantages |
|
|
Notaden bennetti frog bioadhesives |
Protein-based elastic glue |
Bone adhesion and fragments fixation (cartilage bone repair) Binding to biological tissues as well as other surfaces |
Better biocompatibility and biodegradation than fibrin glues Function in moist environments |
Lower adhesion strength than cyanoacrylates |
|
Phosphate-functionalised and amino acid-based polyester copolymers |
Bovine bone adhesion (orthopaedic) Scaffold materials for spinal cord injury Mesh grafts to treat hernias, ulcers and burns |
Adhesion strength of 1.17 MPa Biodegradable in vitro and in vivo Higher interface compliance |
Cohesive failure Low curing kinetics and adhesive properties on translationally relevant substrates |
|
|
Polyacrylamide-based copolymer with hydroxyl and hexyl groups |
Repeatable and robust underwater adhesion to various substrates Material transfer, temporary fixation (orthopaedics) and material separation Bovine bone adhesion |
Tensile shear strength of 2 MPa Enhanced toughness and cohesion strength Good elastic properties Rapid and reversible adhesion in water |
Poor adhesion to bovine bone approx. 363 kPa Low mechanical strength |
|
|
Adhesives based on complex interaction between different proteins |
Strong attachment to inorganic/organic surfaces at dry/wet environment Reliable crosslinking using oxidation agents, such as iron Suitable for joining titanium implants to a bone and/or bonding sternal bones |
Non-immunogenicity and low cytotoxicity Greater adhesion on various substrates with adhesion strength of up to 10 MPa Good biodegradability Low exothermic reaction for the bonding of sternal bones |
Difficulties relating to protein extraction resulting in high production costs, hampering the practical use Further research needed to determine the suitability of this adhesive as bone adhesive |
|
|
Polyphenolic protein and phosphoserine-based adhesive |
Strong attachment in a wet environment Reconstruction of craniofacial fractures Bonding of wet bone fragments Bond tissues to metallic and polymeric biomaterials |
Maximum adhesion strength and hardness in <30 s Osteointegration, bone ingrowth and resorbability Small amount of adhesive needed to achieve the optimal properties Biodegradable and osteoconductive |
Further in vitro and in vivo studies need to be conducted to verify the suitability to natural bone adhesion |
|
5. Bioadhesives for Bone Fracture Repair
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering9060250
References
- Augat, P.; Simon, U.; Liedert, A.; Claes, L. Mechanics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporos. Int. 2005, 16 (Suppl. 2), 36–43.
- Gupta, H.S.; Zioupos, P. Fracture of bone tissue: The ‘hows’ and the ‘whys’. Med. Eng. Phys. 2008, 30, 1209–1226.
- Nellans, K.W.; Kowalski, E.; Chung, K.C. The Epidemiology of Distal Radius Fractures. Hand Clin. 2012, 28, 113–125.
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Health Longev. 2021, 2, e580–e592.
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140.
- Kovach, T.K.; Dighe, A.S.; Lobo, P.I.; Cui, Q. Interactions between MSCs and immune cells: Implications for bone healing. J. Immunol. Res. 2015, 248.
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50.
- Elliott, D.S.; Newman, K.J.H.; Forward, D.P.; Hahn, D.M.; Ollivere, B.; Kojima, K.; Handley, R.; Rossiter, N.D.; Wixted, J.J.; Smith, R.M.; et al. A unified theory of bone healing and nonunion. Bone Jt. J. 2016, 98B, 884–891.
- Jalil, M.A.A.; Shuid, A.N.; Muhammad, N. Role of medicinal plants and natural products on osteoporotic fracture healing. Evid.-Based Complement. Altern. Med. 2012, 2012, 714512.
- Vriens, J.; Moos, K. Morbidity of the infraorbital nerve following orbitozygomatic complex fractures. J. Cranio-Maxillofac. Surg. 1995, 23, 363–368.
- Muraoka, M.; Nakai, Y. Twenty years of statistics and observation of facial bone fracture. Acta Oto-Laryngol. Suppl. 1998, 118, 261–265.
- Kasimova, G.; Yunusov, D.; Sakhibova, M.; Yaxudaev, E.; Batirova, B. Analysis of fracture of the foot bones in children according to the andijan region. Ann. Rom. Soc. Cell Biol. 2021, 25, 6186–6192.
- Meena, S.; Sharma, P.; Sambharia, A.K.; Dawar, A. Fractures of distal radius: An overview. J. Fam. Med. Prim. Care 2014, 3, 325–332.
- Souyris, F.; Klersy, F.; Jammet, P.; Payrot, C. Malar bone fractures and their sequelae. J. Cranio-Maxillofac. Surg. 1989, 17, 64–68.
- Welling, R.D.; Jacobson, J.A.; Jamadar, D.A.; Chong, S.; Caoili, E.M.; Jebson, P.J.L. MDCT and radiography of wrist fractures: Radiographic sensitivity and fracture patterns. Am. J. Roentgenol. 2008, 190, 10–16.
- Jones, W.A.; Ghorbal, M.S. Fractures of the trapezium a report on three cases. J. Hand Surg. Am. 1985, 10, 227–230.
- Erol, B.; Tanrikulu, R.; Görgün, B. Maxillofacial fractures. Analysis of demographic distribution and treatment in 2901 patients (25-year experience). J. Cranio-Maxillofac. Surg. 2004, 32, 308–313.
- Hogg, N.J.V.; Stewart, T.C.; Armstrong, J.E.A.; Girotti, M.J. Epidemiology of maxillofacial injuries at trauma hospitals in Ontario, Canada, between 1992 and 1997. J. Trauma Inj. Infect. Crit. Care 2000, 49, 425–432.
- Hwang, K.; You, S.H. Analysis of facial bone fractures: An 11-year study of 2,094 patients. Indian J. Plast. Surg. 2010, 43, 42–48.
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17(12), 1726–1733.
- Fisher, W.D.; Hamblen, D.L. Problems and pitfalls of compression fixation of long bone fractures: A review of results and complications. Injury 1979, 10, 99–107.
- Larsson, S. Treatment of osteoporotic fractures. Scandinavian J. Surg. 2002, 91, 140–146.
- Xue, X.H.; Yan, S.G.; Cai, X.Z.; Shi, M.M.; Lin, T. Intramedullary nailing versus plating for extra-articular distal tibial metaphyseal fracture: A systematic review and meta-analysis. Injury 2014, 45, 667–676.
- Misra, A.; Kapur, R.; Maffulli, N. Complex proximal humeral fractures in adults- A systematic review of management. Injury 2001, 32, 363–372.
- Gross, C.E.; Nunley, J.A. Navicular Stress Fractures. Foot Ankle Int. 2015, 36, 1117–1122.
- Jackson, L.C.; Pacchiana, P.D. Common complications of fracture repair. Clin. Tech. Small Anim. Pract. 2004, 19, 168–179.
- Uhthoff, H.K.; Poitras, P.; Backman, D.S. Internal plate fixation of fractures: Short history and recent developments. J. Orthop. Sci. 2006, 11, 118–126.
- Mucha, P.E.; Farnell, M.B. Analysis of Pelvic Fracture Management. J. Trauma Inj. Infect. Crit. 1984, 24, 379–386.
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 1–19.
- Petrie, E.M. Cyanoacrylate adhesives in surgical applications: A critical review. Rev. Adhes. Adhes. 2014, 2, 253–310.
- Chen, C.F.; Shen, H.H.; Lin, T.Y.; Yu, Y.J.; Chen, W.C.; Chu, I.M. Studies on the preparation and characterization of mPEG-polyester biodegradable bioglue for bone defect repair. J. Med. Biol. Eng. 2011, 31, 13–17.
- Arora, M.; Chan, E.K.S.; Gupta, S.; Diwan, A.D. Polymethylmethacrylate bone cements and additives: A review of the literature. World J. Orthop. 2013, 4, 67–74.
- Schlag, G.; Redl, H. Fibrin Sealant in Orthopedic Surgery. Clin. Orthop. Relat. Res. 1988, 227, 269–285.
- Sánchez-Fernández, M.J.; Hammoudeh, H.; Lanao, R.P.F.; van Erk, M.; van Hest, J.C.M.; Leeuwenburgh, S.C.G. Bone-Adhesive Materials: Clinical Requirements, Mechanisms of Action, and Future Perspective. Adv. Mater. Interfaces 2019, 6, 1802021.
- Dunne, N.J.; Orr, J.F. Thermal characteristics of curing acrylic bone cement. ITBM-RBM 2001, 22, 88–97.
- Gosain, A.K. The current status of tissue glues: I. For bone fixation. Plast. Reconstr. Surg. 2002, 109, 2581–2583.
- Kandalam, U.; Bouvier, A.; Casas, S.; Smith, R.; Gallego, A.; Rothrock, J.; Thompson, J.; Huang, C.-Y.; Stelnicki, E. Novel bone adhesives: A comparison of bond strengths in vitro. Int. J. Oral Maxillofac. Surg. 2013, 42, 1054–1059.
- Kukleta, J.F.; Freytag, C.; Weber, M. Efficiency and safety of mesh fixation in laparoscopic inguinal hernia repair using n-butyl cyanoacrylate: Long-term biocompatibility in over 1, 300 mesh fixations. Hernia 2012, 16, 153–162.
- Lee, Y.J.; Jung, G.B.; Choi, S.; Lee, G.; Kim, J.H.; Son, H.S.; Bae, H.; Park, H.-K. Biocompatibility of a novel cyanoacrylate based tissue adhesive: Cytotoxicity and biochemical property evaluation. PLoS ONE 2013, 8, e79761.
- Pascual, G.; Sotomayor, S.; Rodríguez, M.; Pérez-Köhler, B.; Kühnhardt, A.; Fernández-Gutiérrez, M.; Román, J.S.; Bellón, J.M. Cytotoxicity of cyanoacrylate-based tissue adhesives and short-term preclinical in vivo biocompatibility in abdominal hernia repair. PLoS ONE 2016, 11, e0157920.
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive glasses: Where are we and where are we going? J. Funct. Biomater. 2018, 9, 25.
- Zhang, M.; Liu, J.; Zhu, T.; Le, H.; Wang, X.; Guo, J.; Liu, G.; Ding, J. Functional Macromolecular Adhesives for Bone Fracture Healing. ACS Appl. Mater. Interfaces 2021, 14, 1–19.
- Xu, L.; Gao, S.; Zhou, R.; Zhou, F.; Qiao, Y.; Qiu, D. Bioactive Pore-Forming Bone Adhesives Facilitating Cell Ingrowth for Fracture Healing. Adv. Mater. 2020, 32, 1–7.
- Shahbazi, S.; Moztarzadeh, F.; Sadeghi, G.M.M.; Jafari, Y. In vitro study of a new biodegradable nanocomposite based on poly propylene fumarate as bone glue. Mater. Sci. Eng. C 2016, 69, 1201–1209.
- Brandt, J.; Henning, S.; Michler, G.; Hein, W.; Bernstein, A.; Schulz, M. Nanocrystalline hydroxyapatite for bone repair: An animal study. J. Mater. Sci. Mater. Med. 2009, 21, 283–294.
- Schreader, K.J.; Bayer, I.S.; Milner, D.J.; Loth, E.; Jasiuk, I. A polyurethane-based nanocomposite biocompatible bone adhesive. J. Appl. Polym. Sci. 2012, 127, 4974–4982.
- Bhagat, V.; Becker, M.L. Degradable Adhesives for Surgery and Tissue Engineering. Biomacromolecules 2017, 18, 3009–3039.
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558.
- Brandeis, E.; Katz, D.; Silbermann, M.; Zinman, C. A new bioadhesive for in vivo bone adhesion. J. Mater. Sci. Mater. Med. 1993, 4, 543–546.
- Böker, K.O.; Richter, K.; Jäckle, K.; Taheri, S.; Grunwald, I.; Borcherding, K.; von Byern, J.; Hartwig, A.; Wildemann, B.; Schilling, A.F.; et al. Current state of bone adhesives-Necessities and hurdles. Materials 2019, 12, 3975.
- Jain, R.; Wairkar, S. Recent developments and clinical applications of surgical glues: An overview. Int. J. Biol. Macromol. 2019, 137, 95–106.
- Idris, S.B.; Arvidson, K.; Plikk, P.; Ibrahim, S.; Finne-Wistrand, A.; Albertsson, A.-C.; Bolstad, A.I.; Mustafa, K. Polyester copolymer scaffolds enhance expression of bone markers in osteoblast-like cells. J. Biomed. Mater. Res. Part A 2010, 9999A, 631–639.
- Kobayashi, H.; Hyon, S.-H.; Ikada, Y. Water-curable and biodegradable prepolymers. J. Biomed. Mater. Res. 1991, 25, 1481–1494.
- Shi, Y.; Zhou, P.; Jérôme, V.; Freitag, R.; Agarwal, S. Enzymatically Degradable Polyester-Based Adhesives. ACS Biomater. Sci. Eng. 2015, 1, 971–977.
- Annabi, N.; Yue, K.; Tamayol, A.; Khademhosseini, A. Elastic sealants for surgical applications. Eur. J. Pharm. Biopharm. 2015, 95, 27–39.
- Wang, Y.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606.
- Vogt, L.; Ruther, F.; Salehi, S.; Boccaccini, A.R. Poly(Glycerol Sebacate) in Biomedical Applications—A Review of the Recent Literature. Adv. Healthc. Mater. 2021, 10, 2002026.
- Ferrari, P.F.; Aliakbarian, B.; Lagazzo, A.; Tamayol, A.; Palombo, D.; Perego, P. Tailored electrospun small-diameter graft for vascular prosthesis. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 635–643.
- Kalakonda, P.; Aldhahri, M.A.; Abdel-Wahab, M.S.; Tamayol, A.; Moghaddam, K.M.; Ben Rached, F.; Pain, A.; Khademhosseini, A.; Memic, A.; Chaieb, S. Microfibrous silver-coated polymeric scaffolds with tunable mechanical properties. RSC Adv. 2017, 7, 34331–34338.
- Memic, A.; Aldhahri, M.; Tamayol, A.; Mostafalu, P.; Abdel-Wahab, M.S.; Samandari, M.; Moghaddam, K.M.; Annabi, N.; Bencherif, S.A.; Khademhosseini, A. Nanofibrous silver-coated polymeric scaffolds with tunable electrical properties. Nanomaterials 2017, 7, 63.
- Saudi, A.; Rafienia, M.; Kharazi, A.Z.; Salehi, H.; Zarrabi, A.; Karevan, M. Design and fabrication of poly (glycerol sebacate)-based fibers for neural tissue engineering: Synthesis, electrospinning, and characterization. Polym. Adv. Technol. 2019, 30, 1427–1440.
- Sha, D.; Wu, Z.; Zhang, J.; Ma, Y.; Yang, Z.; Yuan, Y. Development of modified and multifunctional poly(glycerol sebacate) (PGS)-based biomaterials for biomedical applications. Eur. Polym. J. 2021, 161, 110830.
- Wu, T.; Frydrych, M.; Kelly, K.O.; Chen, B. Poly(glycerol sebacate urethane)—Cellulose Nanocomposites with Water-Active Shape-Memory Effects. Biomacromolecules 2014, 15, 2663–2671.
- Zhao, X.; Wu, Y.; Du, Y.; Chen, X.; Lei, B.; Xue, Y.; Ma, P.X. A highly bioactive and biodegradable poly(glycerol sebacate)-silica glass hybrid elastomer with tailored mechanical properties for bone tissue regeneration. J. Mater. Chem. B 2015, 3, 3222–3233.
- Kerativitayanan, P.; Tatullo, M.; Khariton, M.; Joshi, P.; Perniconi, B.; Gaharwar, A.K. Gaharwar, Nanoengineered Osteoinductive and Elastomeric Scaffolds for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2017, 3, 590–600.
- Yu, S.; Shi, J.; Liu, Y.; Si, J.; Yuan, Y.; Liu, C. A mechanically robust and flexible PEGylated poly(glycerol sebacate)/β-TCP nanoparticle composite membrane for guided bone regeneration. J. Mater. Chem. B 2019, 7, 3279–3290.
- Hasandoost, L.; Rodriguez, O.; Alhalawani, A.; Zalzal, P.; Schemitsch, E.H.; Waldman, S.D.; Papini, M.; Towler, M.R. The role of poly(methyl methacrylate) in management of bone loss and infection in revision total knee arthroplasty: A review. J. Funct. Biomater. 2020, 11, 25.
- Farrar, D.F. Bone adhesives for trauma surgery: A review of challenges and developments. Int. J. Adhes. Adhes. 2012, 33, 89–97.
- Lu, J. Orthopedic bone cements. Biomechanics and Biomaterials in Orthopedics, 2nd ed.; Springer: London, UK, 2016; pp. 123–138.
- Mikkelsen, D.B.; Pedersen, C.; Højbjerg, T.; Schønheyder, H.C. Culture of multiple peroperative biopsies and diagnosis of infected knee arthroplasties. APMIS 2006, 114, 449–452.
- Sa, Y.; Wang, M.; Deng, H.; Wang, Y.; Jiang, T. Beneficial effects of biomimetic nano-sized hydroxyapatite/antibiotic gentamicin enriched chitosan-glycerophosphate hydrogel on the performance of injectable polymethylmethacrylate. RSC Adv. 2015, 5, 91082–91092.
- Grossterlinden, L.; Janssen, A.; Schmitz, N.; Priemel, M.; Pogoda, P.; Amling, M.; Rueger, J.M.; Linhart, W. Deleterious tissue reaction to an alkylene bis(dilactoyl)-methacrylate bone adhesive in long-term follow up after screw augmentation in an ovine model. Biomaterials 2006, 27, 3379–3386.
- Meyer, G.; Muster, D.; Schmitt, D.; Jung, P.; Jaeger, J.H. Bone bonding through bioadhesives: Present status. Biomater. Med. Devices. Artif. Organs 1979, 7, 55–71.
- Heiss, C.; Kraus, R.; Peters, F.; Henn, W.; Schnabelrauch, M.; Berg, A.; Pautzsch, T.; Weisser, J.; Schnettler, R. Development of a bioresorbable self-hardening bone adhesive based on a composite consisting of polylactide methacrylates and β-tricalcium phosphate. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90B, 55–66.
- Jensen, M.E.; Evans, A.J.; Mathis, J.M.; Kallmes, D.F.; Cloft, H.J.; Dion, J.E. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: Technical aspects. Am. J. Neuroradiol. 1997, 18, 1897–1904.
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705.
- Shah, N.V.; Meislin, R. Current state and use of biological adhesives in orthopedic surgery. Orthopedics 2013, 36, 945–956.
- Millar, N.L.; Bradley, T.A.; Walsh, N.A.; Appleyard, R.C.; Tyler, M.J.; Murrell, G.A.C. Frog glue enhances rotator cuff repair in a laboratory cadaveric model. J. Shoulder Elb. Surg. 2009, 18, 639–645.
- Bhagat, V.; O’Brien, E.; Zhou, J.; Becker, M.L. Caddisfly Inspired Phosphorylated Poly(ester urea)-Based Degradable Bone Adhesives. Biomacromolecules 2016, 17, 3016–3024.
- Duarte, A.P.; Coelho, J.F.; Bordado, J.C.; Cidade, M.T.; Gil, M.H. Surgical adhesives: Systematic review of the main types and development forecast. Prog. Polym. Sci. 2011, 37, 1031–1050.
- Kirillova, A.; Kelly, C.; von Windheim, N.; Gall, K. Bioinspired Mineral-Organic Bioresorbable Bone Adhesive. Adv. Healthc. Mater. 2018, 7, e1800467.
- Yamamoto, H.; Nagai, A.; Okada, T.; Nishida, A. Synthesis and adhesive studies of barnacle model proteins. Mar. Chem. 1989, 26, 331–338.
- Nishida, J.; Higaki, Y.; Takahara, A. Synthesis and characterization of barnacle adhesive mimetic towards underwater adhesion. Chem. Lett. 2015, 44, 1047–1049.
- Fan, H.; Wang, J.; Gong, J.P. Barnacle Cement Proteins-Inspired Tough Hydrogels with Robust, Long-Lasting, and Repeatable Underwater Adhesion. Adv. Funct. Mater. 2020, 31, 2009334.
- Silverman, H.G.; Roberto, F.F. Understanding marine mussel adhesion. Mar. Biotechnol. 2007, 9, 661–681.
- Flammang, P.; Lambert, A.; Bailly, P.; Hennebert, E. Polyphosphoprotein-containing marine adhesives. J. Adhes. 2009, 85, 447–464.
- Yamamoto, H.; Kuno, S.; Nagai, A.; Nishida, A.; Yamauchi, S.; Ikeda, K. Insolubilizing and adhesive studies of water-soluble synthetic model proteins. Int. J. Biol. Macromol. 1990, 12, 305–310.
- Meredith, H.J.; Jenkins, C.L.; Wilker, J.J. Enhancing the adhesion of a biomimetic polymer yields performance rivaling commercial glues. Adv. Funct. Mater. 2014, 24, 3259–3267.
- Li, A.; Jia, M.; Mu, Y.; Jiang, W.; Wan, X. Humid bonding with a water-soluble adhesive inspired by mussels and sandcastle worms. Macromol. Chem. Phys. 2014, 216, 450–459.
- Bhagat, V.; O’Brien, E.; Zhou, J.; Becker, M.L. A novel class of injectable bioceramics that glue tissues and biomaterials. Materials 2018, 11, 2492.
- Shao, H.; Bachus, K.N.; Stewart, R.J. A water-borne adhesive modeled after the sandcastle glue of P. californica. Macromol. Biosci. 2009, 9, 464–471.
- Gardziella, A.; Mueller, R. Phenolic Resins. Kunstst. Ger. Plast. 1990, 80, 66–68.
- Donkerwolcke, M.; Burny, F.; Muster, D. Tissues and bone adhesives historical aspects. Biomaterials 1998, 19, 1461–1466.
- Ishihara, K.; Nakabayashi, N. Adhesive bone cement both to bone and metals: 4-META in MMA initiated with tri-n-butyl borane. J. Biomed. Mater. Res. 1989, 23, 1475–1482.
- Wistlich, L.; Rücker, A.; Schamel, M.; Kübler, A.C.; Gbureck, U.; Groll, J. A Bone Glue with Sustained Adhesion under Wet Conditions. Adv. Healthc. Mater. 2017, 6, 1600902.
- Liu, X.; Pujari-Palmer, M.; Wenner, D.; Procter, P.; Insley, G.; Engqvist, H. Adhesive cements that bond soft tissue ex vivo. Materials 2019, 12, 2473.
- Hulsart-Billström, G.; Stelzl, C.; Procter, P.; Pujari-Palmer, M.; Insley, G.; Engqvist, H.; Larsson, S. In vivo safety assessment of a bio-inspired bone adhesive. J. Mater. Sci. Mater. Med. 2020, 31, 24.
- Le Nihouannen, D.; Saffarzadeh, A.; Gauthier, O.; Moreau, F.; Pilet, P.; Spaethe, R.; Layrolle, P.; Daculsi, G. Bone tissue formation in sheep muscles induced by a biphasic calcium phosphate ceramic and fibrin glue composite. J. Mater. Sci. Mater. Med. 2008, 19, 667–675.
- Cassaro, C.V.; Justulin, L.A., Jr.; De Lima, P.R.; Golim, M.D.A.; Biscola, N.P.; de Castro, M.V.; De Oliveira, A.L.R.; Doiche, D.P.; Pereira, E.J.; Ferreira, R.S., Jr.; et al. Fibrin biopolymer as scaffold candidate to treat bone defects in rats. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190027.
- Kumbar, S.G.; Toti, U.S.; Deng, M.; James, R.; Laurencin, C.T.; Aravamudhan, A.; Harmon, M.; Ramos, D.M. Novel mechanically competent polysaccharide scaffolds for bone tissue engineering. Biomed. Mater. 2011, 6, 065005.
- Hoffmann, B.; Volkmer, E.; Kokott, A.; Augat, P.; Ohnmacht, M.; Sedlmayr, N.; Schieker, M.; Claes, L.; Mutschler, W.; Ziegler, G. Characterisation of a new bioadhesive system based on polysaccharides with the potential to be used as bone glue. J. Mater. Sci. Mater. Med. 2009, 20, 2001–2009.
- Choi, B.-H.; Cheong, H.; Ahn, J.-S.; Zhou, C.; Kwon, J.J.; Cha, H.J.; Jun, S.H. Engineered mussel bioglue as a functional osteoinductive binder for grafting of bone substitute particles to accelerate in vivo bone regeneration. J. Mater. Chem. B 2015, 3, 546–555.
- Brown, M.; Kay, G.W.; Cochran, D.; Fiorellini, J.; Hess, B. From bench-to-bedside: Licensing and development of a mineral-organic bone adhesive for bone repair. In Society for Biomaterials Annual Meeting and Exposition 42nd Annual Meeting; Society for Biomaterials: Mount Laurel, NJ, USA, 2019; Volume 40, 169p.
- Bystrom, J.L.; Pujari-Palmer, M. Phosphoserine functionalized cements preserve metastable phases, and reprecipitate octacalcium phosphate, hydroxyapatite, dicalcium phosphate, and amorphous calcium phosphate, during degradation, in vitro. J. Funct. Biomater. 2019, 10, 54.
