Researchers are increasingly paying attention to sustainable methods for storing energy. Many researchers are now concentrating their efforts on the development and exploration of novel materials for use in energy storage devices due to the limited supply of existing energy sources such as oil, coal, and natural gas, and escalating regional tensions. Because of these issues, sustainable renewable energy sources have been touted as an alternative to nonrenewable fuels. Deployment of renewable energy sources requires efficient and reliable energy storage devices due to their intermittent nature. High-performance electrochemical energy storage technologies with high power and energy densities are heralded to be the next-generation storage devices. Transition metal chalcogenides (TMCs) have sparked interest among electrode materials because of their intriguing electrochemical properties.
- Chalcogenides
- electrochemical
- energy storage
- Batteries
- Supercapacitors
1. Batteries
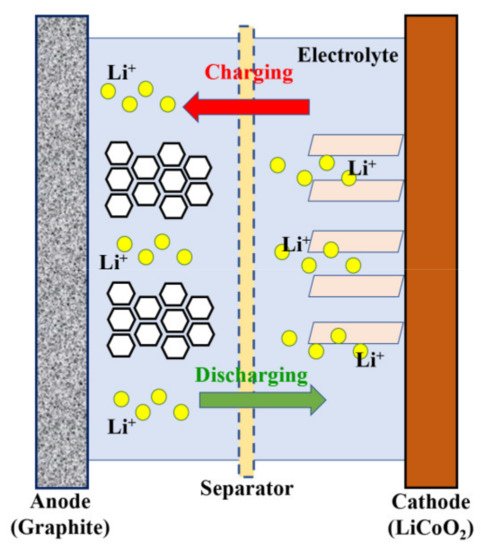
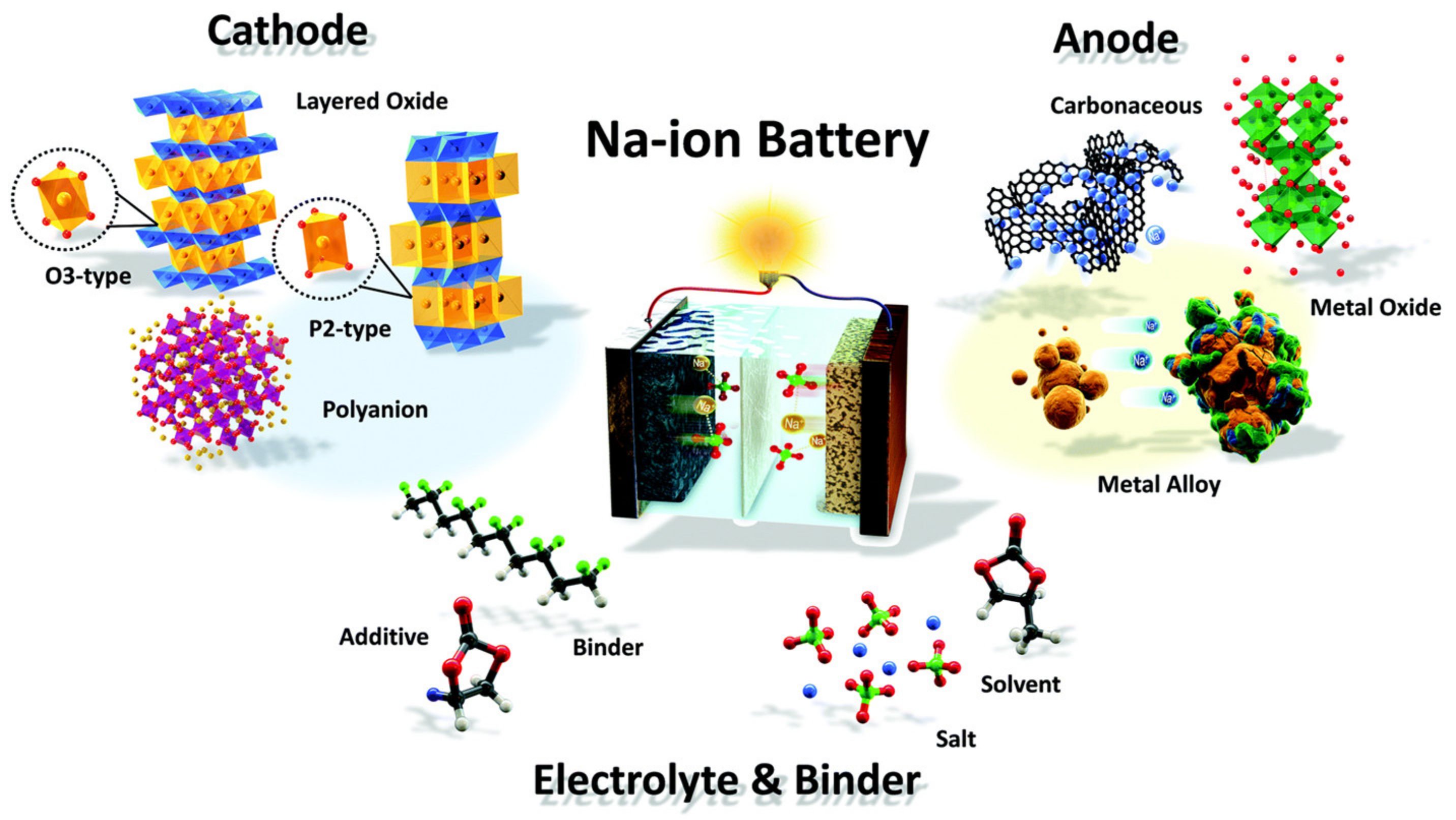
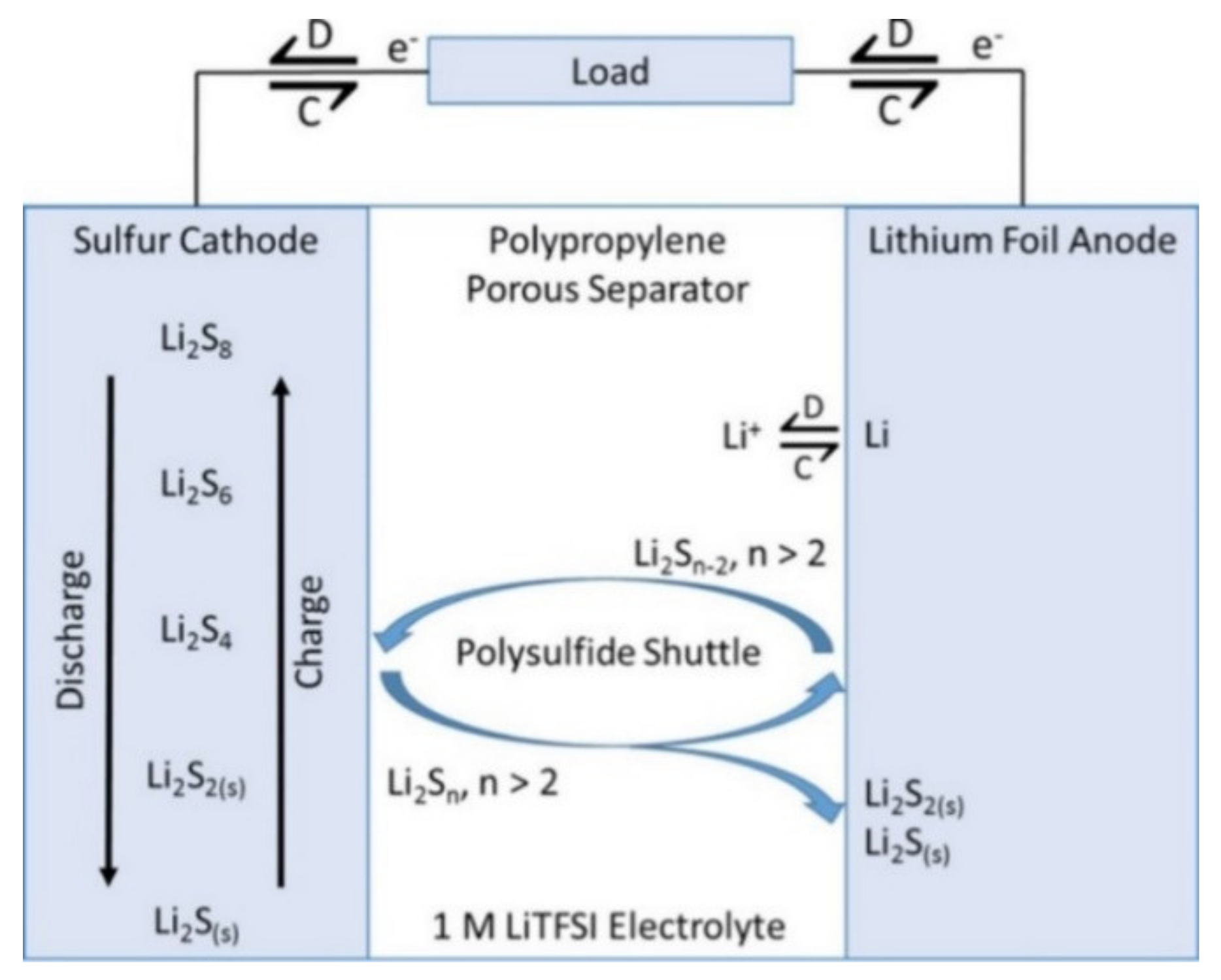
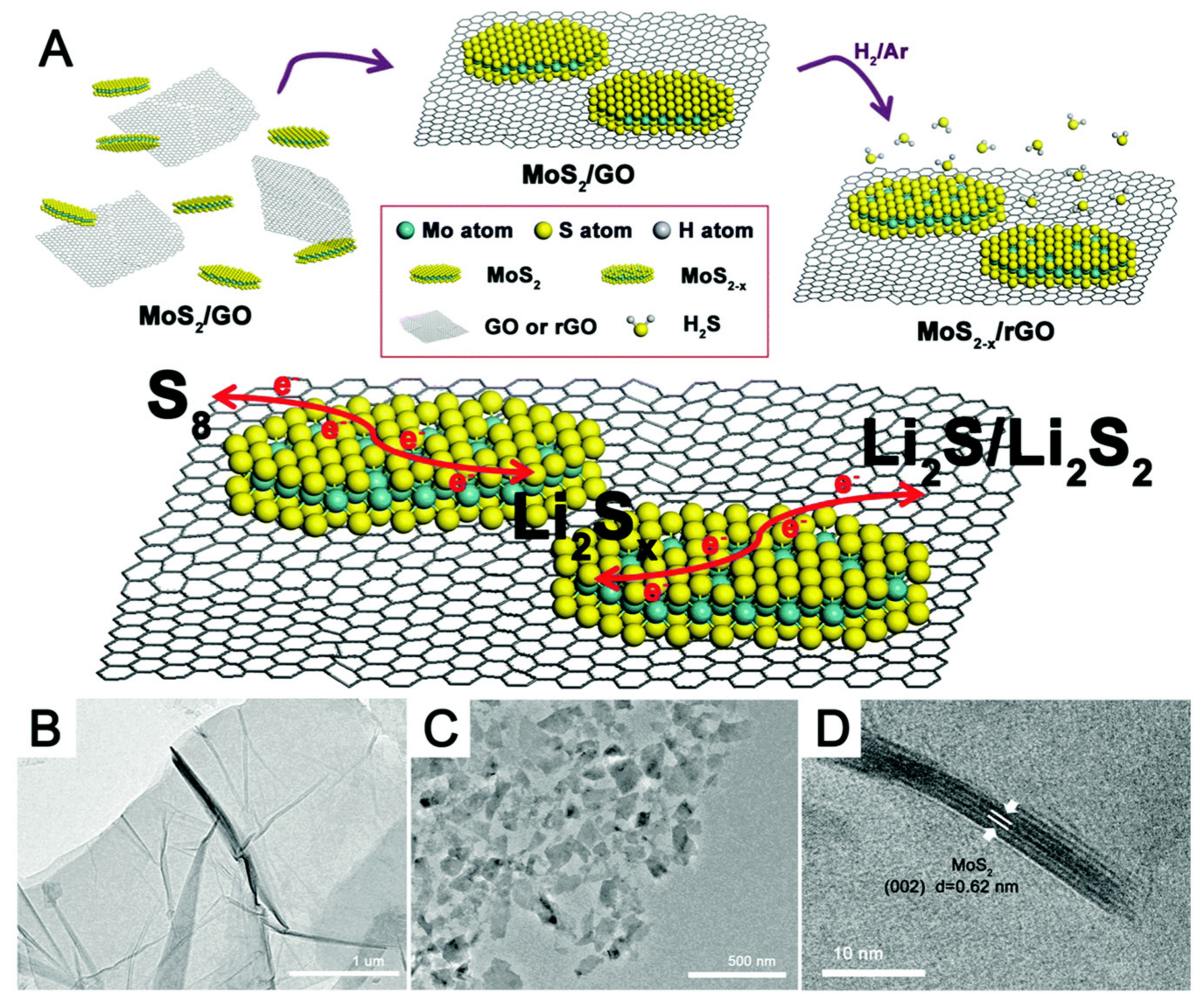
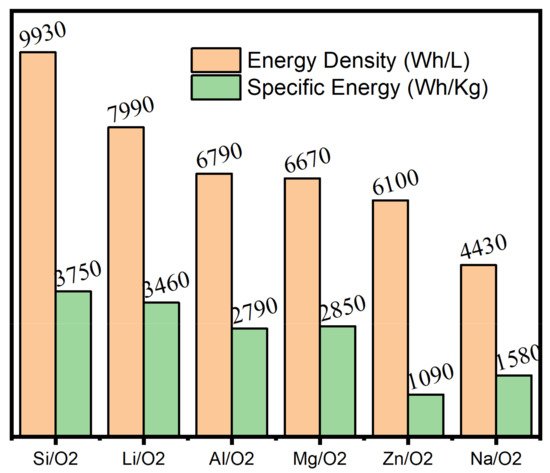
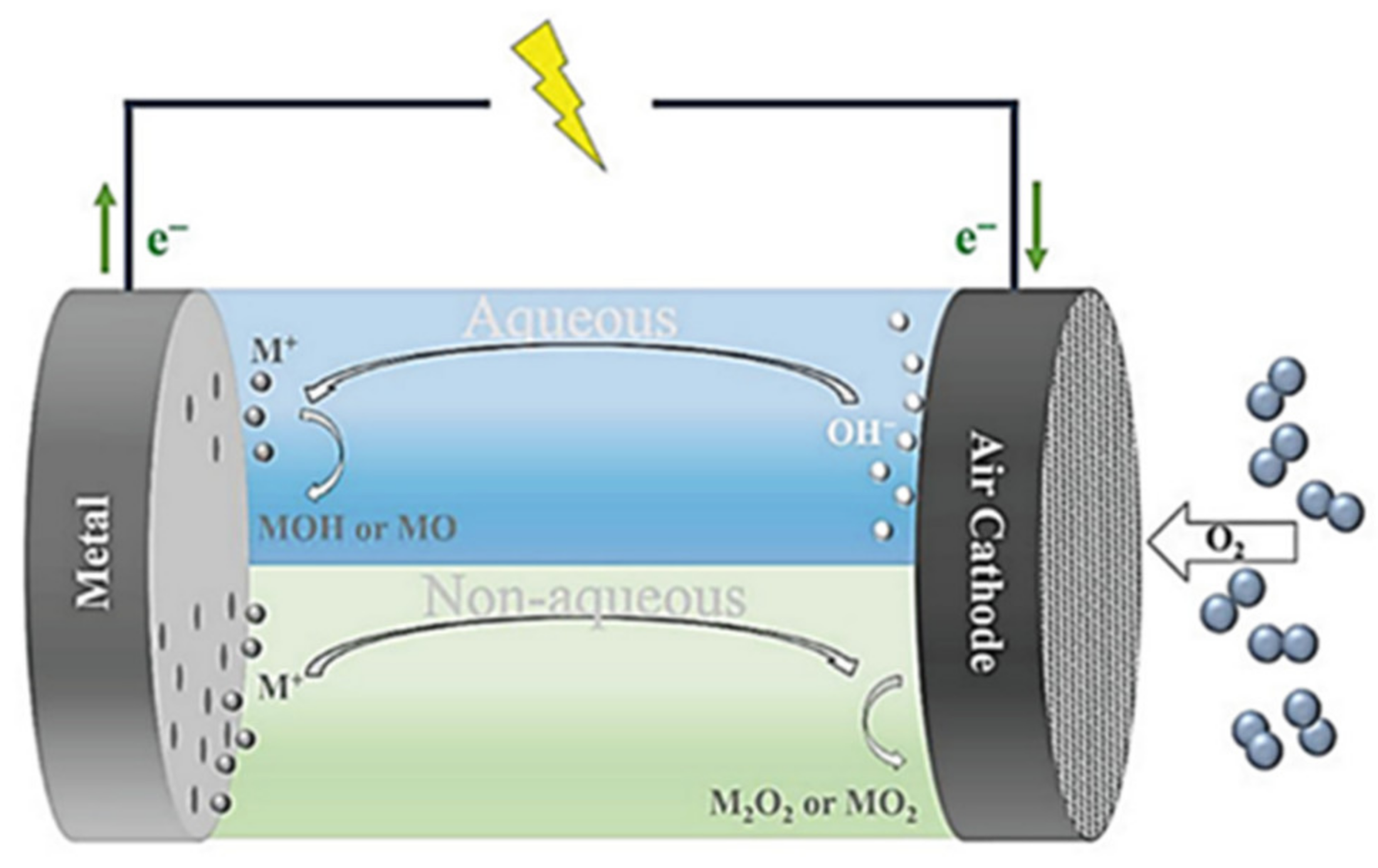
2. Supercapacitors
This entry is adapted from the peer-reviewed paper 10.3390/en15114052
References
- Zhang, H.; Li, X.; Liu, W.; Yue, H.; Shi, Z.; Yin, Y.; Yang, S. Olivine LiFePO4 as an additive into LiCoO2 electrodes for LIBs to improve high-voltage performances. J. Alloys Compd. 2021, 869, 159188.
- Abraham, K.M. Intercalation positive electrodes for rechargeable sodium cells. Solid State Ionics 1982, 7, 199–212.
- Rajabathar, J.R.; Al-lohedan, H.A.; Arunachalam, P.; Issa, Z.A.; Gnanamani, M.K.; Appaturi, J.N.; Ibrahim, S.N.; Mohammed Dahan, W. Unexpected discovery of low-cost maricite NaFePO4 as a high-performance electrode for Na-ion batteries. J. Alloys Compd. 2021, 850, 540–545.
- Rajabathar, J.R.; Al-lohedan, H.A.; Arunachalam, P.; Issa, Z.A.; Gnanamani, M.K.; Appaturi, J.N.; Ibrahim, S.N.; Mohammed Dahan, W. Challenges for Na-ion negative electrodes. J. Alloys Compd. 2021, 850, A1011.
- Rajabathar, J.R.; Al-lohedan, H.A.; Arunachalam, P.; Issa, Z.A.; Gnanamani, M.K.; Appaturi, J.N.; Ibrahim, S.N.; Mohammed Dahan, W. Sodium-Ion Batteries. J. Alloys Compd. 2021, 850, 947–958.
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614.
- Nagde, K.R.; Dhoble, S.J. Li-S ion batteries: A substitute for Li-ion storage batteries. In Energy Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 335–371.
- Evers, S.; Yim, T.; Nazar, L.F. Understanding the nature of absorption/adsorption in nanoporous polysulfide sorbents for the Li–S battery. J. Phys. Chem. C 2012, 116, 19653–19658.
- Moy, D.; Narayanan, S.R. Mixed Conduction Membranes Suppress the Polysulfide Shuttle in Lithium-Sulfur Batteries. J. Electrochem. Soc. 2017, 164, A560–A566.
- Moy, D.; Manivannan, A.; Narayanan, S.R. Direct Measurement of Polysulfide Shuttle Current: A Window into Understanding the Performance of Lithium-Sulfur Cells. J. Electrochem. Soc. 2014, 162, A1–A7.
- Li, H.; Tsai, C.; Koh, A.L.; Cai, L.; Contryman, A.W.; Fragapane, A.H.; Zhao, J.; Han, H.S.; Manoharan, H.C.; Abild-Pedersen, F.; et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 2016, 15, 48–53.
- Ji, G.; Yu, Y.; Yao, Q.; Qu, B.; Chen, D.; Chen, W.; Xie, J.; Lee, J.Y. Promotion of reversible Li+ storage in transition metal dichalcogenides by Ag nanoclusters. NPG Asia Mater. 2016, 8, e247.
- Lin, H.; Yang, L.; Jiang, X.; Li, G.; Zhang, T.; Yao, Q.; Zheng, G.W.; Lee, J.Y. Electrocatalysis of polysulfide conversion by sulfur-deficient MoS2 nanoflakes for lithium–sulfur batteries. Energy Environ. Sci. 2017, 10, 1476–1486.
- Clark, S.; Latz, A.; Horstmann, B. A Review of Model-Based Design Tools for Metal-Air Batteries. Batteries 2018, 4, 5.
- Zou, X.; Lu, Q.; Liao, K.; Shao, Z. Towards practically accessible aprotic Li-air batteries: Progress and challenges related to oxygen-permeable membranes and cathodes. Energy Storage Mater. 2022, 45, 869–902.
- Lu, S.-H.; Lu, H.-C. Pouch-type hybrid Li-air battery enabled by flexible composite lithium-ion conducting membrane. J. Power Source 2021, 489, 229431.
- Parveen, N.; Khan, Z.; Ansari, S.A.; Park, S.; Senthilkumar, S.T.; Kim, Y.; Ko, H.; Cho, M.H. Feasibility of using hollow double walled Mn2O3 nanocubes for hybrid Na-air battery. Chem. Eng. J. 2019, 360, 415–422.
- Wang, H.; Xu, Q. Materials Design for Rechargeable Metal-Air Batteries. Matter 2019, 1, 565–595.
- Hou, X.; Zhang, Y.; Cui, C.; Lin, C.; Li, Y.; Bu, D.; Yan, G.; Liu, D.; Wu, Q.; Song, X.-M. Photo-assisted Al-air batteries based on gel-state electrolyte. J. Power Source 2022, 533, 231377.
- Wu, P.; Zhao, Q.; Yu, H.; Tang, Z.; Li, Y.; Huang, D.; Sun, D.; Wang, H.; Tang, Y. Modification on water electrochemical environment for durable Al-Air Battery: Achieved by a Low-Cost sucrose additive. Chem. Eng. J. 2022, 438, 135538.
- Zhang, L.; Shao, Q.; Zhang, J. An overview of non-noble metal electrocatalysts and their associated air cathodes for Mg-air batteries. Mater. Rep. Energy 2021, 1, 100002.
- Vaghefinazari, B.; Snihirova, D.; Wang, C.; Wang, L.; Deng, M.; Höche, D.; Lamaka, S.V.; Zheludkevich, M.L. Exploring the effect of sodium salt of Ethylenediaminetetraacetic acid as an electrolyte additive on electrochemical behavior of a commercially pure Mg in primary Mg-air batteries. J. Power Source 2022, 527, 231176.
- Leong, K.W.; Wang, Y.; Ni, M.; Pan, W.; Luo, S.; Leung, D.Y.C. Rechargeable Zn-air batteries: Recent trends and future perspectives. Renew. Sustain. Energy Rev. 2022, 154, 111771.
- Qian, M.; Guo, M.; Qu, Y.; Xu, M.; Liu, D.; Hou, C.; Isimjan, T.T.; Yang, X. Energy barrier engineering of oxygen reduction reaction synergistically promoted by binary Zn-Cu pair sites for advanced Zn–air batteries. J. Alloys Compd. 2022, 907, 164527.
- Hang, B.T.; Watanabe, T.; Egashira, M.; Watanabe, I.; Okada, S.; Yamaki, J. The effect of additives on the electrochemical properties of Fe/C composite for Fe/air battery anode. J. Power Source 2006, 155, 461–469.
- Hang, B.T.; Hayashi, H.; Yoon, S.-H.; Okada, S.; Yamaki, J. Fe2O3-filled carbon nanotubes as a negative electrode for an Fe–air battery. J. Power Source 2008, 178, 393–401.
- Inoishi, A.; Ida, S.; Uratani, S.; Okano, T.; Ishihara, T. High capacity of an Fe–air rechargeable battery using LaGaO3-based oxide ion conductor as an electrolyte. Phys. Chem. Chem. Phys. 2012, 14, 12818–12822.
- Lyu, Z.; Zhou, Y.; Dai, W.; Cui, X.; Lai, M.; Wang, L.; Huo, F.; Huang, W.; Hu, Z.; Chen, W. Recent advances in understanding of the mechanism and control of Li 2 O 2 formation in aprotic Li–O2 batteries. Chem. Soc. Rev. 2017, 46, 6046–6072.
- Geng, D.; Ding, N.-N.; Hor, T.S.A.; Chien, S.W.; Liu, Z.; Zong, Y. Cobalt sulfide nanoparticles impregnated nitrogen and sulfur co-doped graphene as bifunctional catalyst for rechargeable Zn–air batteries. RSC Adv. 2015, 5, 7280–7284.
- Olabi, A.G.; Sayed, E.T.; Wilberforce, T.; Jamal, A.; Alami, A.H.; Elsaid, K.; Rahman, S.M.; Shah, S.K.; Abdelkareem, M.A. Metal-Air Batteries—A Review. Energies 2021, 14, 7373.
- Bao, S.-J.; Li, C.M.; Guo, C.-X.; Qiao, Y. Biomolecule-assisted synthesis of cobalt sulfide nanowires for application in supercapacitors. J. Power Source 2008, 180, 676–681.
- Subramanian, A.; Punnoose, D.; Raman, V.; Gopi, C.V.V.M.; Rao, S.S.; Khan, M.A.; Kim, H.-J. Layer by layer approach to enhance capacitance using metal sulfides for supercapacitor applications. Mater. Lett. 2018, 231, 64–67.
- Sajedi-Moghaddam, A.; Saievar-Iranizad, E.; Pumera, M. Two-dimensional transition metal dichalcogenide/conducting polymer composites: Synthesis and applications. Nanoscale 2017, 9, 8052–8065.
- Kim, Y.; Park, T.; Na, J.; Yi, J.W.; Kim, J.; Kim, M.; Bando, Y.; Yamauchi, Y.; Lin, J. Layered transition metal dichalcogenide/carbon nanocomposites for electrochemical energy storage and conversion applications. Nanoscale 2020, 12, 8608–8625.
- Cherusseri, J.; Choudhary, N.; Sambath Kumar, K.; Jung, Y.; Thomas, J. Recent trends in transition metal dichalcogenide based supercapacitor electrodes. Nanoscale Horiz. 2019, 4, 840–858.
- Ahmad, M.; Hussain, I.; Nawaz, T.; Li, Y.; Chen, X.; Ali, S.; Imran, M.; Ma, X.; Zhang, K. Comparative study of ternary metal chalcogenides (MX.; M= Zn–Co–Ni; X= S, Se, Te): Formation process, charge storage mechanism and hybrid supercapacitor. J. Power Source 2022, 534, 231414.
- Teli, A.M.; Beknalkar, S.A.; Mane, S.M.; Bhat, T.S.; Kambale, B.B.; Patil, S.B.; Sadale, S.B.; Shin, J.C. Electrodeposited crumpled MoS2 nanoflakes for asymmetric supercapacitor. Ceram. Int. 2022, in press.
- Sharma, G.K.; Ranjan, B.; Kaur, D. Electrochemical kinetics of 2D-MoS2 sputtered over stainless-steel mesh: Insights into the Na+ ions storage for flexible supercapacitors. Ceram. Int. 2022, in press.
- Gao, Y.-P.; Huang, K.-J.; Wu, X.; Hou, Z.-Q.; Liu, Y.-Y. MoS2 nanosheets assembling three-dimensional nanospheres for enhanced-performance supercapacitor. J. Alloys Compd. 2018, 741, 174–181.
- Thanh, T.D.; Chuong, N.D.; Van Hien, H.; Kshetri, T.; Kim, N.H.; Lee, J.H. Recent advances in two-dimensional transition metal dichalcogenides-graphene heterostructured materials for electrochemical applications. Prog. Mater. Sci. 2018, 96, 51–85.
- Yang, X.; Zhao, L.; Lian, J. Arrays of hierarchical nickel sulfides/MoS2 nanosheets supported on carbon nanotubes backbone as advanced anode materials for asymmetric supercapacitor. J. Power Source 2017, 343, 373–382.
- Sun, T.; Li, Z.; Liu, X.; Ma, L.; Wang, J.; Yang, S. Facile construction of 3D graphene/MoS2 composites as advanced electrode materials for supercapacitors. J. Power Source 2016, 331, 180–188.
- Ray, S.K.; Pant, B.; Park, M.; Hur, J.; Lee, S.W. Cavity-like hierarchical architecture of WS2/α-NiMoO4 electrodes for supercapacitor application. Ceram. Int. 2020, 46, 19022–19027.
- Li, Y.; Wang, H.; Shu, T.; Yuan, J.; Lu, G.; Lin, B.; Gao, Z.; Wei, F.; Ma, C.; Qi, J.; et al. Two-dimensional hierarchical MoS2 lamella inserted in CoS2 flake as an advanced supercapacitor electrode. J. Energy Storage 2022, 51, 104299.
- Bhol, P.; Swain, S.; Altaee, A.; Saxena, M.; Samal, A.K. Cobalt–iron decorated tellurium nanotubes for high energy density supercapacitor. Mater. Today Chem. 2022, 24, 100871.
- Rathore, H.K.; Hariram, M.; Ganesha, M.K.; Singh, A.K.; Das, D.; Kumar, M.; Awasthi, K.; Sarkar, D. Charge storage mechanism in vanadium telluride/carbon nanobelts as electroactive material in an aqueous asymmetric supercapacitor. J. Colloid Interface Sci. 2022, 621, 110–118.
- Theerthagiri, J.; Karuppasamy, K.; Durai, G.; Rana, A.U.H.S.; Arunachalam, P.; Sangeetha, K.; Kuppusami, P.; Kim, H.-S. Recent Advances in Metal Chalcogenides (MX; X = S, Se) Nanostructures for Electrochemical Supercapacitor Applications: A Brief Review. Nanomaterials 2018, 8, 256.
- Miao, C.; Xia, G.; Zhu, K.; Ye, K.; Wang, Q.; Yan, J.; Cao, D.; Gong, F.; Wang, G. Enhanced supercapacitor performance of bimetallic metal selenides via controllable synergistic engineering of composition. Electrochim. Acta 2021, 370, 137802.
- Lei, H.; Zhou, J.; Zhao, R.; Peng, H.; Xu, Y.; Wang, F.; Hamouda, H.A.; Zhang, W.; Ma, G. Design and assembly of a novel asymmetric supercapacitor based on all-metal selenides electrodes. Electrochim. Acta 2020, 363, 137206.
- Amiri, M.; Saeed Hosseiny Davarani, S.; Ebrahim Moosavifard, S.; Fu, Y.-Q. Cobalt-molybdenum selenide double-shelled hollow nanocages derived from metal-organic frameworks as high performance electrodes for hybrid supercapacitor. J. Colloid Interface Sci. 2022, 616, 141–151.
- Liu, Q.; Hong, X.; You, X.; Zhang, X.; Zhao, X.; Chen, X.; Ye, M.; Liu, X. Designing heterostructured metal sulfide core-shell nanoneedle films as battery-type electrodes for hybrid supercapacitors. Energy Storage Mater. 2020, 24, 541–549.
- Wang, Q.; Qu, Z.; Chen, S.; Zhang, D. Metal organic framework derived P-doping with sulfide defect to boost high-performance asymmetric supercapacitors. J. Colloid Interface Sci. 2022, in press.
- Lu, L.; Xu, Q.; Chen, Y.; Zhou, Y.; Jiang, T.; Zhao, Q. Preparation of metal sulfide electrode materials derived based on metal organic framework and application of supercapacitors. J. Energy Storage 2022, 49, 104073.
