Historically, vitamin C has been associated with many regulatory processes that involve specific signaling pathways. Among the most studied signaling pathways are those involved in the regulation of aging, differentiation, neurotransmission, proliferation, and cell death processes in cancer. This wide variety of regulatory effects is due to the fact that vitamin C has a dual mechanism of action. On the one hand, it regulates the expression of genes associated with proliferation (Ccnf and Ccnb1), differentiation (Sox-2 and Oct-4), and cell death (RIPK1 and Bcl-2). At the same time, vitamin C can act as a regulator of kinases, such as MAPK and p38, or by controlling the activation of the NF-kB pathway, generating chronic responses related to changes in gene expression or acute responses associated with the regulation of signal transduction processes. To date, data from the literature show a permanent increase in processes regulated by vitamin C.
1. Introduction
The reduced form of vitamin C (ascorbic acid, AA) is an essential micronutrient of small size; it is soluble in water and has two dissociable protons with pKa values of 4.2 and 11.8. At physiological pH, its reduced form predominates as the monovalent ascorbate anion (AA); when it loses the second proton, it is oxidized to dehydroascorbic acid (DHA) [
1,
2,
3]. Most mammals can synthesize vitamin C from D-glucose in the liver, except guinea pigs, bats, and higher primates, including humans, due to the absence of the enzyme L-gulonolactone oxidase, which catalyzes the last step of the biosynthesis of vitamin C [
4]. Therefore, to meet the body’s requirements, vitamin C must be incorporated into the diet [
1]. The best-known function of vitamin C is as an antioxidant agent that can act as a cofactor of enzymatic reactions involved in the synthesis of catecholamines, carnitine, cholesterol, amino acids, and some hormonal peptides, as well as in the maintenance of brain function and the protection of central nervous system (CNS) structures [
1,
3,
5,
6,
7].
AA uptake in different cells is performed by the sodium-ascorbate cotransporters SVCT1 and SVCT2, which stereospecifically transport the reduced form of vitamin C, L-ascorbate [
8,
9,
10,
11]. Vitamin C can also be transported in its oxidized form, DHA, through the facilitative glucose transporters GLUT1, GLUT2, GLUT3, GLUT4, and GLUT8 [
12,
13,
14,
15,
16]. However, for a long time, it has been postulated that the contribution of DHA to the accumulation of vitamin C in tissues is relatively low [
3,
17,
18,
19].
2. Molecular Pathways Regulated by Vitamin C
One of the first targets for vitamin C was discovered via its relationship to the NF‒κB pathway. In this pathway, vitamin C has an inhibitory function; in studies carried out in endothelial cells, millimolar doses of AA inhibited NF-κB and IL-8 activation in response to tumor necrosis factor (TNF) [
20]. In this study, the authors also evaluated the toxicity generated by high doses of vitamin C supplementation and did not detect cell damage or lipid peroxidation [
20,
21]. Furthermore, they were able to determine that the inhibition of the NF-κB pathway was not due to the antioxidant activity of vitamin C, but rather to the direct inhibition of IκB kinase α/β (IKKα/β) [
20,
21,
22,
23]. In line with this notion, IKKα/β is a kinase responsible for the phosphorylation of IκBα protein that maintains NF-κB-p65 in the cytoplasm [
24]. IκBα phosphorylation is a signal for proteasomal degradation of this protein, allowing NF-κB-p65 nuclear translocation (
Figure 1A), triggering the activation of specific genes [
24,
25,
26]. In line with these findings, it was postulated that AA is a regulator of IKKα/β activity; however, subsequent studies determined that AA has no action on IKKα/β [
22,
27]. Interestingly, it was shown that DHA was a regulator of IKKα/β mediated by directly binding to this kinase, inhibiting it, and finally controlling the activity of NF-κB [
27]. This function of DHA was determined through immunoprecipitation experiments using p-IκBα-GST where derivatives of vitamin C, AA, DHA, oxalic acid, and threonic acid were used. Only treatment with DHA inhibited IκBα-phosphorylation, and this inhibition was mediated by DHA directly blocking the activity of IKKα/β and p38, likely competing for the binding of ATP to the active site of IKKβ [
22,
27]. Given this evidence, it was concluded that vitamin C has a dual action against reactive oxygen species (ROS). Intracellularly, AA would fulfill its antioxidant function by neutralizing ROS, generating DHA. Thus, the intracellular accumulation of DHA would block the activation of NF-κB, involving vitamin C in signaling processes that control inflammatory responses and cell death among others (
Figure 1A).
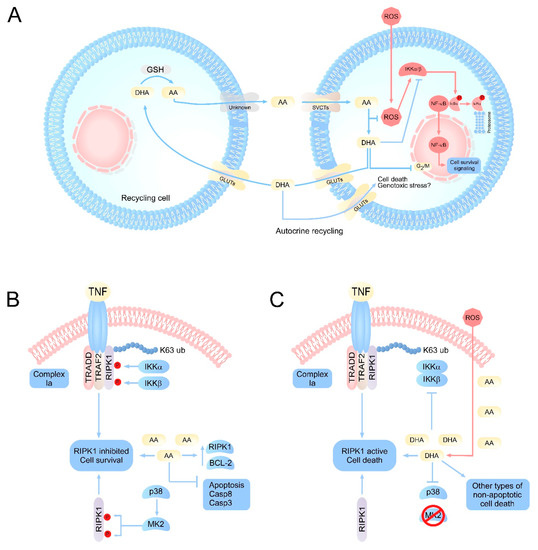
Figure 1. Integrative vision of the principal molecular pathways regulated by vitamin C. (A) Scheme of vitamin C recycling in normal cells or cells with oxidative stress. Under normal conditions, AA concentrations remain homeostatically stable due to the efficient recycling of DHA by specialized cells. However, under conditions of oxidative stress or inefficient recycling, an accumulation of intracellular DHA can occur. DHA would target the inhibition of IKK α/β, metabolic enzymes such as GAPDH, as well as the production of genotoxic stress, resulting in the induction of cell death. (B) Intracellular effects of vitamin C on signaling pathways associated with cell death. The physiological levels of AA would have a protective function intracellularly, favoring the inhibition of apoptosis by inducing overexpression of antiapoptotic genes, as well as caspases. At the same time, AA could maintain RIPK1 in its inhibited state, which favors cell survival. (C) Under pathophysiological or acute oxidative stress conditions, ROS overload induces a massive oxidation of AA to DHA, intracellularly. The accumulation of DHA results in the inhibition of IKK α/β, and p38, which can trigger the activation of RIPK1 and cell death due to necroptosis, in cells that accumulate high concentrations of vitamin C, such as neurons. AA: ascorbic acid; DHA: dehydroascorbic acid; SVCT2s: sodium-dependent vitamin C transporter; GLUTs: glucose transporters; RIPK1: receptor-interacting serine/threonine-protein kinase 1; MK2: p38MAPK-activated protein kinase 2; TRADD: TNFR1-associated death domain protein; TRAF2: TNF receptor associated factor 2.
Another kinase-dependent pathway that is regulated by vitamin C is that of the mitogen-activated protein kinases (MAPK), which involves three other MAPK-dependent pathways, extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinases (JNK), and p38 kinase, which are involved in proliferation, differentiation, and apoptosis, respectively [
10,
28,
29]. The first studies examining the relationship between vitamin C and MAPK concluded that vitamin C was involved in in vitro cell death processes, but the mechanism of action was unknown. Thus, the possible regulation of MAPK-ERK mediated by vitamin C was analyzed. For this, leukemia cell lines were treated with AA (0-500 µM) for 1 to 3 h in order to analyze ERK activation by in vitro phosphorylation assays. In cells treated with concentrations as low as 100 μM AA, phosphorylation of ERK and therefore activation was induced [
10,
30]. Thus, it was proposed that the regulation of ERK mediated by vitamin C would be associated with eventual apoptotic processes that are observed in certain tumor lines when treated with vitamin C because ERK activation is associated with proliferative processes and cell death. However, to date, it has been shown that the use of pharmacological doses of AA induces tumor death from conventional necrosis due to the extracellular generation of H
2O
2 [
31,
32], as discussed in detail later. At the same time, it has also been shown that AA can antagonize apoptosis in cancer cells induced by classical mechanisms, such as treatment with doxorubidicin, TRAIL, or FAS [
33,
34,
35]. In line with this notion, treatment with physiological doses of AA in neuronal cultures induces overexpression of antiapoptotic genes, such as Bcl-2, and decreases the expression of proapoptotic genes, such as Bax and caspase 8 [
19]. Thus, the current evidence suggests that physiological doses of AA could inhibit apoptosis rather than activate this death pathway. Furthermore, AA-mediated ERK activation could be associated with neuronal arborization mechanisms, which would be an indicator of neuronal “good health” [
10].
This entry is adapted from the peer-reviewed paper 10.3390/antiox10020215