The precise monitoring of environmental contaminants and agricultural plant stress factors, respectively responsible for damages to our ecosystems and crop losses, has become nowadays a topic of uttermost importance. This is also highlighted by the recent introduction of the so-called "Sustainable Development Goals" of the United Nations, which aim at reducing pollutants while implementing more sustainable food production practices leading to a reduced impact on all ecosystems. In this context, the standard methods currently used in these fields represent a sub-optimal solution, being expensive, laboratory-based techniques, and typically requiring trained personnel with high expertise. Recent advances in both biotechnology and material science, have led to the emergence of new sensing (and biosensing) technologies, enabling low-cost, precise, and real-time detection. An especially interesting category of biosensors is represented by field-effect transistor-based biosensors (bio-FETs), which enable the possibility of performing in-situ, continuous, selective, and sensitive measurements of a wide palette of different parameters of interest. Furthermore, bio-FETs offer the possibility of being fabricated using innovative and sustainable materials, employing various device configurations, each customized for a specific application. In the specific field of environmental and agricultural monitoring, the exploitation of these devices is particularly attractive as it paves the way to early detection and intervention strategies useful to limit, or even to completely avoid negative outcomes (such as diseases to animals or ecosystems losses).
- bio-FETs
- environmental pollutants
- plant stresses
- transistors
1. Bio-FET Operation and Configurations
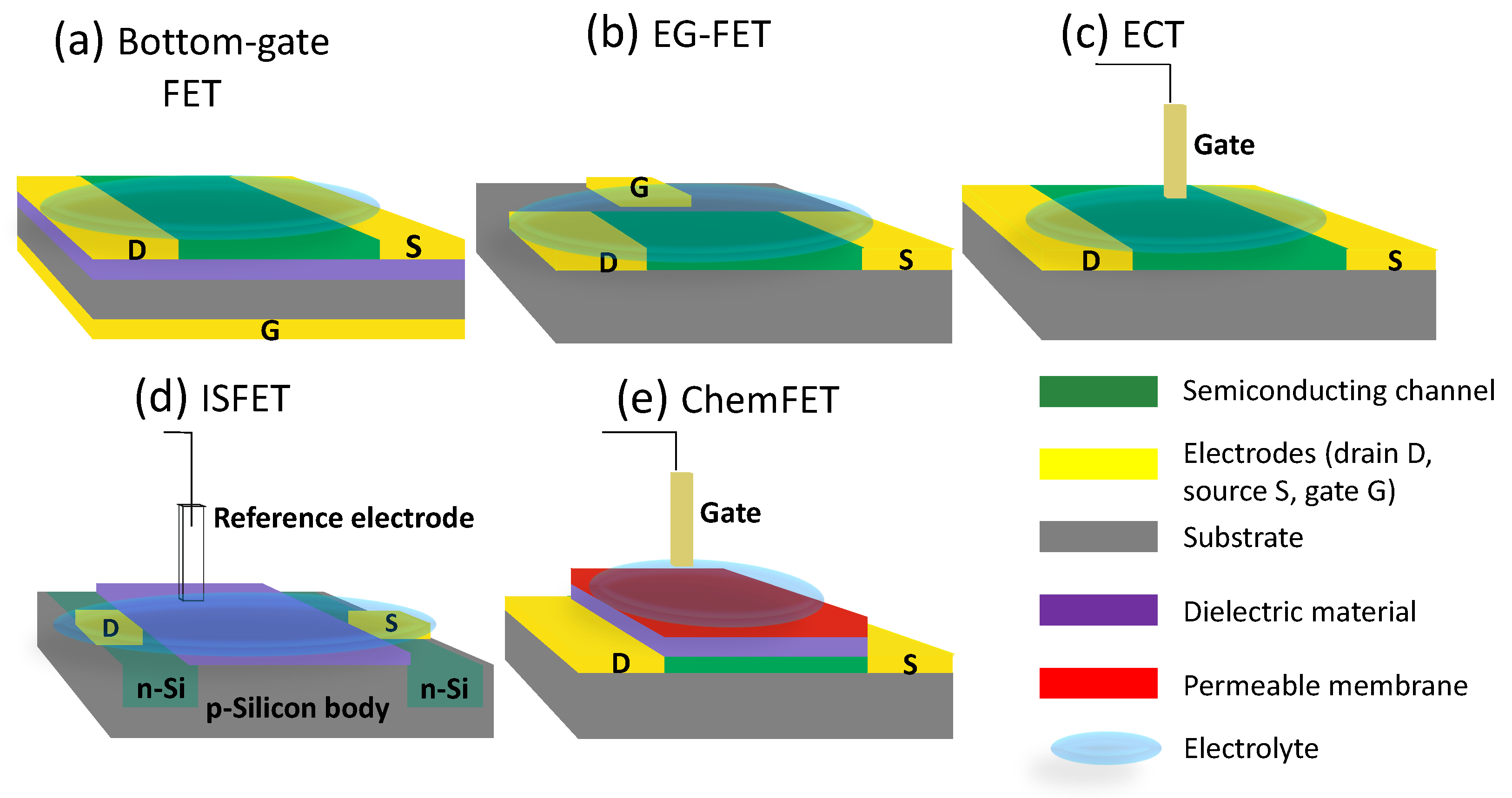
2. Bio-FETs in Agricultural Plants Applications
2.1. Abiotic Stresses
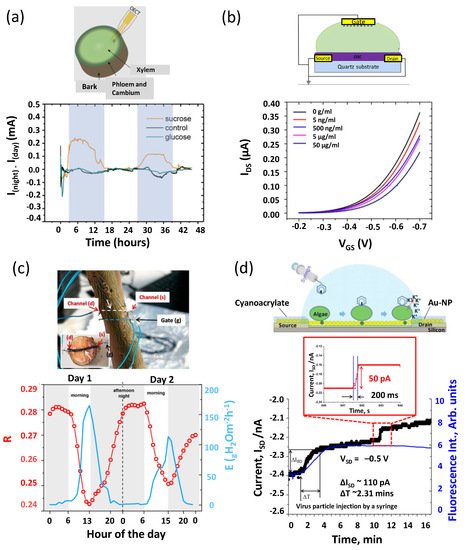
2.2. Biotic Stresses
4.3. Plant Metabolites and pH Measurements
This entry is adapted from the peer-reviewed paper 10.3390/s22114178
References
- Shkodra, B.; Petrelli, M.; Costa Angeli, M.A.; Garoli, D.; Nakatsuka, N.; Lugli, P.; Petti, L. Electrolyte-gated carbon nanotube field-effect transistor-based biosensors: Principles and applications. Appl. Phys. Rev. 2021, 8, 041325.
- Wang, D.; Noël, V.; Piro, B. Electrolytic gated organic field-effect transistors for application in biosensors—A Review. Electronics 2016, 5, 9.
- Golio, M.; Golio, J. RF and Microwave Passive and Active Technologies; CRC Press: Boca Raton, FL, USA, 2018.
- Lee, M.L.; Fitzgerald, E.A.; Bulsara, M.T.; Currie, M.T.; Lochtefeld, A. Strained Si, SiGe, and Ge channels for high-mobility metal-oxide-semiconductor field-effect transistors. J. Appl. Phys. 2005, 97, 011101.
- Lüssem, B.; Riede, M.; Leo, K. Doping of organic semiconductors. Phys. Status Solidi 2013, 210, 9–43.
- Petti, L.; Münzenrieder, N.; Vogt, C.; Faber, H.; Büthe, L.; Cantarella, G.; Bottacchi, F.; Anthopoulos, T.D.; Tröster, G. Metal oxide semiconductor thin-film transistors for flexible electronics. Appl. Phys. Rev. 2016, 3, 021303.
- Zhao, Y.; Guo, Y.; Liu, Y. 25th anniversary article: Recent advances in n-type and ambipolar organic field-effect transistors. Adv. Mater. 2013, 25, 5372–5391.
- Zhang, S. Review of modern field effect transistor technologies for scaling. J. Phys. Conf. Ser. 2020, 1617, 012054.
- Kuo, Y. Thin film transistor technology—Past, present, and future. Electrochem. Soc. Interface 2013, 22, 55.
- Myronov, M. Molecular Beam Epitaxy of High Mobility Silicon, Silicon Germanium and Germanium Quantum Well Heterostructures. In Molecular Beam Epitaxy; Elsevier: Amsterdam, The Netherlands, 2018; pp. 37–54.
- De Moraes, A.C.M.; Kubota, L.T. Recent trends in field-effect transistors-based immunosensors. Chemosensors 2016, 4, 20.
- Benda, V.; Grant, D.A.; Gowar, J. Discrete and Integrated Power Semiconductor Devices: Theory and Applications; John Wiley & Sons: Hoboken, NJ, USA, 1999.
- Li, H.; Shi, W.; Song, J.; Jang, H.J.; Dailey, J.; Yu, J.; Katz, H.E. Chemical and biomolecule sensing with organic field-effect transistors. Chem. Rev. 2018, 119, 3–35.
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018, 3, 17086.
- Bergveld, P. Thirty years of ISFETOLOGY: What happened in the past 30 years and what may happen in the next 30 years. Sens. Actuators B Chem. 2003, 88, 1–20.
- Polk, B.J. ChemFET arrays for chemical sensing microsystems. In Proceedings of the SENSORS, 2002 IEEE, Orlando, FL, USA, 12–14 June 2002; Volume 1, pp. 732–735.
- Winie, T.; Arof, A.K.; Thomas, S. Polymer Electrolytes: Characterization Techniques and Energy Applications; John Wiley & Sons: Hoboken, NJ, USA, 2019.
- Enderby, J.; Neilson, G. The structure of electrolyte solutions. Rep. Prog. Phys. 1981, 44, 593.
- Scuratti, F.; Bonacchini, G.E.; Bossio, C.; Salazar-Rios, J.M.; Talsma, W.; Loi, M.A.; Antognazza, M.R.; Caironi, M. Real-Time Monitoring of Cellular Cultures with Electrolyte-Gated Carbon Nanotube Transistors. ACS Appl. Mater. Interfaces 2019, 11, 37966–37972.
- Joshi, S.; Bhatt, V.D.; Rani, H.; Becherer, M.; Lugli, P. Understanding the influence of in-plane gate electrode design on electrolyte gated transistor. Microelectron. Eng. 2018, 199, 87–91.
- Oldham, K.B. A Gouy–Chapman–Stern model of the double layer at a (metal)/(ionic liquid) interface. J. Electroanal. Chem. 2008, 613, 131–138.
- Le Gall, J.; Vasilijević, S.; Battaglini, N.; Mattana, G.; Noël, V.; Brayner, R.; Piro, B. Algae-functionalized hydrogel-gated organic field-effect transistor. Application to the detection of herbicides. Electrochim. Acta 2021, 372, 137881.
- Bhatt, V.D.; Joshi, S.; Becherer, M.; Lugli, P. Flexible, low-cost sensor based on electrolyte gated carbon nanotube field effect transistor for organo-phosphate detection. Sensors 2017, 17, 1147.
- Berto, M.; Vecchi, E.; Baiamonte, L.; Condò, C.; Sensi, M.; Di Lauro, M.; Sola, M.; De Stradis, A.; Biscarini, F.; Minafra, A.; et al. Label free detection of plant viruses with organic transistor biosensors. Sens. Actuators B Chem. 2019, 281, 150–156.
- Schöning, M.J.; Poghossian, A. Recent advances in biologically sensitive field-effect transistors (BioFETs). Analyst 2002, 127, 1137–1151.
- Pachauri, V.; Ingebrandt, S. Biologically sensitive field-effect transistors: From ISFETs to NanoFETs. Essays Biochem. 2016, 60, 81–90.
- Antonisse, M.M.; Reinhoudt, D.N. Potentiometric anion selective sensors. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 1999, 11, 1035–1048.
- Jiao, Y.; Wang, X.; Chen, Y.; Castellano, M.J.; Schnable, J.C.; Schnable, P.S.; Dong, L. In-Planta Nitrate Detection Using Insertable Plant Microsensor. In Proceedings of the 2019 20th IEEE International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; pp. 37–40.
- Araújo, S.O.; Peres, R.S.; Barata, J.; Lidon, F.; Ramalho, J.C. Characterising the Agriculture 4.0 Landscape—Emerging Trends, Challenges and Opportunities. Agronomy 2021, 11, 667.
- Kalsoom, T.; Ramzan, N.; Ahmed, S.; Ur-Rehman, M. Advances in sensor technologies in the era of smart factory and industry 4.0. Sensors 2020, 20, 6783.
- Almalki, F.A.; Soufiene, B.O.; Alsamhi, S.H.; Sakli, H. A low-cost platform for environmental smart farming monitoring system based on IoT and UAVs. Sustainability 2021, 13, 5908.
- Munirathinam, S. Industry 4.0: Industrial internet of things (IIOT). In Advances in Computers; Elsevier: Amsterdam, The Netherlands, 2020; Volume 117, pp. 129–164.
- Dillon, T.; Wu, C.; Chang, E. Cloud computing: Issues and challenges. In Proceedings of the 2010 24th IEEE International Conference on Advanced Information Networking and Applications, Perth, Australia, 20–23 April 2010; pp. 27–33.
- Gröger, C. Building an industry 4.0 analytics platform. Datenbank-Spektrum 2018, 18, 5–14.
- Lichtenthaler, H.K. Vegetation stress: An introduction to the stress concept in plants. J. Plant Physiol. 1996, 148, 4–14.
- Brilli, F.; Loreto, F.; Baccelli, I. Exploiting plant volatile organic compounds (VOCs) in agriculture to improve sustainable defense strategies and productivity of crops. Front. Plant Sci. 2019, 10, 264.
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38.
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448.
- Coppedè, N.; Janni, M.; Bettelli, M.; Maida, C.L.; Gentile, F.; Villani, M.; Ruotolo, R.; Iannotta, S.; Marmiroli, N.; Marmiroli, M.; et al. An in vivo biosensing, biomimetic electrochemical transistor with applications in plant science and precision farming. Sci. Rep. 2017, 7, 16195.
- Amato, D.; Montanaro, G.; Vurro, F.; Coppedé, N.; Briglia, N.; Petrozza, A.; Janni, M.; Zappettini, A.; Cellini, F.; Nuzzo, V. Towards In Vivo Monitoring of Ions Accumulation in Trees: Response of an in Planta Organic Electrochemical Transistor Based Sensor to Water Flux Density, Light and Vapor Pressure Deficit Variation. Appl. Sci. 2021, 11, 4729.
- Janni, M.; Coppede, N.; Bettelli, M.; Briglia, N.; Petrozza, A.; Summerer, S.; Vurro, F.; Danzi, D.; Cellini, F.; Marmiroli, N.; et al. In Vivo Phenotyping for the Early Detection of Drought Stress in Tomato. Plant Phenom. 2019, 2019, 6168209.
- Diacci, C.; Abedi, T.; Lee, J.W.; Gabrielsson, E.O.; Berggren, M.; Simon, D.T.; Niittylä, T.; Stavrinidou, E. Diurnal in vivo xylem sap glucose and sucrose monitoring using implantable organic electrochemical transistor sensors. Iscience 2021, 24, 101966.
- Lee, S.W.; Lee, E.H.; Thiel, G.; Van Etten, J.L.; Saraf, R.F. Noninvasive measurement of electrical events associated with a single chlorovirus infection of a microalgal cell. ACS Nano 2016, 10, 5123–5130.
- Vurro, F.; Janni, M.; Coppedè, N.; Gentile, F.; Manfredi, R.; Bettelli, M.; Zappettini, A. Development of an In Vivo Sensor to Monitor the Effects of Vapour Pressure Deficit (VPD) Changes to Improve Water Productivity in Agriculture. Sensors 2019, 19, 4667.
- Michela, J.; Claudia, C.; Federico, B.; Sara, P.; Filippo, V.; Nicola, C.; Manuele, B.; Davide, C.; Loreto, F.; Zappettini, A. Real-time monitoring of Arundo donax response to saline stress through the application of in vivo sensing technology. Sci. Rep. 2021, 11, 18598.
- Takemoto, A.; Araki, T.; Uemura, T.; Noda, Y.; Yoshimoto, S.; Izumi, S.; Tsuruta, S.; Sekitani, T. Printable Transparent Microelectrodes toward Mechanically and Visually Imperceptible Electronics. Adv. Intell. Syst. 2020, 2, 2000093.
- Lee, K.; Park, J.; Lee, M.S.; Kim, J.; Hyun, B.G.; Kang, D.J.; Na, K.; Lee, C.Y.; Bien, F.; Park, J.U. In-situ synthesis of carbon nanotube–graphite electronic devices and their integrations onto surfaces of live plants and insects. Nano Lett. 2014, 14, 2647–2654.
- Bischak, C.G.; Flagg, L.Q.; Ginger, D.S. Ion exchange gels allow organic electrochemical transistor operation with hydrophobic polymers in aqueous solution. Adv. Mater. 2020, 32, 2002610.
- Tao, T.; Zhou, Y.; Ma, M.; He, H.; Gao, N.; Cai, Z.; Chang, G.; He, Y. Novel graphene electrochemical transistor with ZrO2/rGO nanocomposites functionalized gate electrode for ultrasensitive recognition of methyl parathion. Sens. Actuators B Chem. 2021, 328, 128936.
- Strand, E.J.; Bihar, E.; Gleason, S.M.; Han, S.; Schreiber, S.W.; Renny, M.N.; Malliaras, G.G.; McLeod, R.R.; Whiting, G.L. Printed Organic Electrochemical Transistors for Detecting Nutrients in Whole Plant Sap. Adv. Electron. Mater. 2021, 8, 2100853.
- Friederich, P.; León, S.; Perea, J.D.; Roch, L.M.; Aspuru-Guzik, A. The influence of sorbitol doping on aggregation and electronic properties of PEDOT: PSS: A theoretical study. Mach. Learn. Sci. Technol. 2020, 2, 01LT01.
- García, J.A.; Glasa, M.; Cambra, M.; Candresse, T. P lum pox virus and sharka: A model potyvirus and a major disease. Mol. Plant Pathol. 2014, 15, 226–241.
- Wang, H.; Wang, Y.; Hou, X.; Xiong, B. Bioelectronic Nose Based on Single-Stranded DNA and Single-Walled Carbon Nanotube to Identify a Major Plant Volatile Organic Compound (p-Ethylphenol) Released by Phytophthora Cactorum Infected Strawberries. Nanomaterials 2020, 10, 479.
- Wang, H.; Ramnani, P.; Pham, T.; Villarreal, C.C.; Yu, X.; Liu, G.; Mulchandani, A. Gas biosensor arrays based on single-stranded DNA-functionalized single-walled carbon nanotubes for the detection of volatile organic compound biomarkers released by huanglongbing disease-infected citrus trees. Sensors 2019, 19, 4795.
- Saraf, N.; Barkam, S.; Peppler, M.; Metke, A.; Vázquez-Guardado, A.; Singh, S.; Emile, C.; Bico, A.; Rodas, C.; Seal, S. Microsensor for limonin detection: An indicator of citrus greening disease. Sens. Actuators B Chem. 2019, 283, 724–730.
- Teklić, T.; Parađiković, N.; Špoljarević, M.; Zeljković, S.; Lončarić, Z.; Lisjak, M. Linking abiotic stress, plant metabolites, biostimulants and functional food. Ann. Appl. Biol. 2021, 178, 169–191.
- Diacci, C.; Lee, J.W.; Janson, P.; Dufil, G.; Méhes, G.; Berggren, M.; Simon, D.T.; Stavrinidou, E. Real-Time Monitoring of Glucose Export from Isolated Chloroplasts Using an Organic Electrochemical Transistor. Adv. Mater. Technol. 2020, 5, 1900262.
- Stitt, M.; Zeeman, S.C. Starch turnover: Pathways, regulation and role in growth. Curr. Opin. Plant Biol. 2012, 15, 282–292.
- Arkhypova, V.; Soldatkin, O.; Mozhylevska, L.; Konvalyuk, I.; Kunakh, V.; Dzyadevych, S. Enzyme biosensor based on pH-sensitive field-effect transistors for assessment of total indole alkaloids content in tissue culture of Rauwolfia serpentina. Electrochem. Sci. Adv. 2021, e2100152.
- Arkhypova, V.N.; Dzyadevych, S.V.; Soldatkin, A.P.; Anna, V.; Martelet, C.; Jaffrezic-Renault, N. Development and optimisation of biosensors based on pH-sensitive field effect transistors and cholinesterases for sensitive detection of solanaceous glycoalkaloids. Biosens. Bioelectron. 2003, 18, 1047–1053.
- Arkhypova, V.N.; Dzyadevych, S.V.; Soldatkin, A.P.; Korpan, Y.I.; Anna, V.; Gravoueille, J.M.; Martelet, C.; Jaffrezic-Renault, N. Application of enzyme field effect transistors for fast detection of total glycoalkaloids content in potatoes. Sens. Actuators B Chem. 2004, 103, 416–422.
- Korpan, Y.I.; Raushel, F.M.; Nazarenko, E.A.; Soldatkin, A.P.; Jaffrezic-Renault, N.; Martelet, C. Sensitivity and specificity improvement of an ion sensitive field effect transistors-based biosensor for potato glycoalkaloids detection. J. Agric. Food Chem. 2006, 54, 707–712.
- Herrmann, V.; Tesche, M. In vivo pH measurement in the xylem of broad-leaved trees using ion-sensitive field-effect transistors. Trees 1992, 6, 13–18.
- Izumi, R.; Ono, A.; Ishizuka, H.; Terao, K.; Takao, H.; Kobayashi, T.; Kataoka, I.; Shimokawa, F. Biological information (pH/EC) sensor device for quantitatively monitoring plant health conditions. In Proceedings of the 2017 IEEE SENSORS, Scotland, UK, 29 October–1 November 2017; pp. 1–3.
