Patients with cystic lesions are at increased risk for developing PDAC. There are several risk factors for developing a cystic precursor lesion and associated PDAC. The relationship of diabetes mellitus and PDAC is an intense area of research interest, with significant progress being made in interactions of diabetes and PDAC development. Further, patients with chronic pancreatitis are at an increased risk of developing a cystic precursor lesion and associated PDAC. Certain genetic syndromes and a familial PDAC have been shown to pose a risk. Based on the literature, we focus on patients with pancreatic cystic lesions, high-risk individuals with a familial PDAC risk, at risk cohorts with pancreatitis, genetic syndromes and germline mutations, and elderly patients with new-onset diabetes.
1. High-Risk Groups Relevant for PDAC Early Detection
Patients with cystic lesions are at increased risk for developing PDAC [
6]. There are several risk factors for developing a cystic precursor lesion and associated PDAC. The relationship of diabetes mellitus and PDAC is an intense area of research interest, with significant progress being made in interactions of diabetes and PDAC development [
7]. Further, patients with chronic pancreatitis are at an increased risk of developing a cystic precursor lesion and associated PDAC [
8]. Certain genetic syndromes and a familial PDAC have been shown to pose a risk [
9]. Based on the literature, we focus on patients with pancreatic cystic lesions, high-risk individuals with a familial PDAC risk, at risk cohorts with pancreatitis, genetic syndromes and germline mutations, and elderly patients with new-onset diabetes (
Table 1).
Table 1. High risk criteria for PDAC.
2. Familial PDAC
A family history of pancreatic cancer is observed in 5% to 10% of patients with PDAC. Gene mutations in CDKN2A (p16), BRCA2, and PALB2 are associated with PDAC [
10,
11,
12,
13]. Familial PDAC is defined as having two or more first-degree relatives with PDAC. The relative risk for PDAC is 2.41 in sporadic cases (i.e., families with only one relative with PDAC or with multiple PDACs in more distant relatives and/or spouses with PDAC), whereas the risk increases to 6.79 and 17.2 times in cases with two and more first-degree relatives with PDAC, respectively [
14]. In the familial PDAC kindreds, risk varied by the number of first-degree relatives with PDAC, such that risk was higher in individuals with three first-degree relatives who had PDAC (Standardized Incidence Ratio, SIR = 17.02; 95% CI = 7.34 to 33.5;
p < 0.01), but lower in individuals who had two first-degree relatives with PDAC (SIR = 3.97, 95%CI = 1.59 to 8.2,
p = 0.05) or with one affected first-degree relative (SIR = 6.86, 95% CI = 3.75 to 11.04,
p < 0.001) [
15]. Whereas risk was higher for familial PDAC kindred members who had one first-degree relative with PDACs compared with two, the confidence intervals for these two estimates largely overlap. Moreover, a higher risk of PDAC has been observed among familial pancreatic cancer kindreds with younger-onset PDAC (age, <50 years; standardized incidence ratio = 9.3%) [
16]. A familial history of pancreatic cancer suggests a high risk for PDAC, and the incidence of PDAC depends on the number of first-degree relative with PDAC. Identification of a family history of PDAC is therefore, important.
3. Inherited Cancer Predisposition Syndromes
Several cancer predisposition syndromes are known to increase PDAC risk (
Table 1) [
17,
18]. Although genetic defects likely remain unknown, several genetic syndromes associated with PDAC have been discovered. The inherited cancer syndromes, such as hereditary breast-ovarian cancer syndrome, hereditary pancreatitis, Peutz–Jeghers syndrome, familial atypical multiple mole myeloma, Lynch syndrome, Li–Fraumeni syndrome, familial breast-ovarian cancer with, and hereditary nonpolyposis colorectal cancer have all been associated with increased risk of developing PDAC [
19,
20,
21,
22,
23]. However, because of their rarity, they in total account for only a small fraction of PDACs.
4. Pancreatic Cystic Lesions
Pancreatic cystic lesions can be divided into three main types: inflammatory, serous and mucinous, as well as other rare cyst types. For the scope of this review, mucinous cystic lesions are described as precursor lesions, harbouring a greater potential for malignancy. It is now well-known that most PDAC originate from microscopic pancreatic intraepithelial neoplasia (PanIN) and macroscopic precursor lesions. PanIN refers to microscopic, intraductal neoplasms (by definition <5 mm) lined by gastric–foveolar epithelia of varying degrees of architectural and cytologic atypia. These cannot be detected by current imaging modalities. While PanINs contribute to most of PDACs, a significant proportion of PDACs arise from macroscopic mucinous neoplasms, such as intraductal papillary mucinous neoplasms (IPMN) and mucinous cystic neoplasms (MCN) (
Figure 1). These cystic precursor lesions share many genetic alterations found in PDACs. Up to 15% of PDACs are thought to arise from mucinous pancreatic cysts, which include IPMNs and MCN [
24]. The incidence of PDAC was reported to be 2% in 349 patients with IPMN who were observed for 3.7 years [
25]. Another study reported that PDAC occurred in 5 of 60 (8%) patients with IPMN (with a diameter <10 mm), who were observed for 87 months, and the 1- and 5-year mortality rates were 1.1% and 6.9%, respectively [
26]. The presence of any pancreatic cyst, including IPMN, was reported to be a high-risk factor for PDAC development, accounting for 0.95% of patients with pancreatic cysts per 1 year, which was 22.5 times higher than that in individuals without pancreatic cystic lesions [
27]. Patients harbouring a cystic lesion are more likely to progress to cancer than even those with family history of PDAC making them the prime target population for screening and surveillance modalities [
16]. However, the matter is complicated by cystic lesions presenting a variable risk of malignant transformation (
Figure 1).
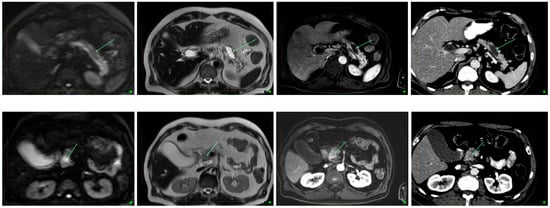
Figure 1. Transformation in IPNM on MRI and CT. From left to right- Diffusion weighted imaging, T2, post contrast T1 and CT images in the same patient. Top row shows a simple cystic lesion consistent with a side branch IPMN, showing no malignant features. Bottom row shows a cystic lesion displaying solid enhancing components with restricted diffusion, consistent with malignant degeneration within an IMPN. Arrows indicate cystic component with no cancer (top), and with cancer (bottom).
IPMNs are macroscopic (>1 cm by definition) cystic tumours characterised by intraductal growth of papillary lesions with typically copious and thick mucin production. IPMNs commonly originate from the main pancreatic duct, its contributing branches, or possible mixed origin. They are more common in elderly men (>65 years). Most patients with IPMNs are asymptomatic; some patients may have nonspecific symptoms of abdominal pain, jaundice, as well as symptoms due to exocrine and/or endocrine pancreatic insufficiency. The pathologic nomenclature of IPMNs is complex and evolving. The incidence and type of invasive malignancy developing in IPMNs differ from one histologic type to another. Pancreatic ductal communication is the key feature differentiating these from other cystic neoplasms. Main duct IPMN is characterized by cystic dilatation of the main duct, variable ductal wall thickening, and thick, abundant mucin that distends and obstructs the duct (
Figure 2). Main duct IPMN have a higher predisposition for malignant transformation, compared with branch duct IPMNs. A longitudinal study by Levy et al. demonstrated the 5-year actuarial risk of progression to high-grade dysplasia among main duct IPMNs was 63%, while 15% in the branch duct IPMNs [
25]. Patients with an IPMN are at an increased risk for developing not only IPMN-associated PDAC but also PDAC independent from the IPMN, concomitant carcinomas [
24]. Existing guidelines recommend immediate surgical resection for any lesion with high-risk stigmata and regular surveillance with interval imaging for all mucinous or indeterminate lesions [
28]. Surveillance protocols vary between the different guidelines [
29].
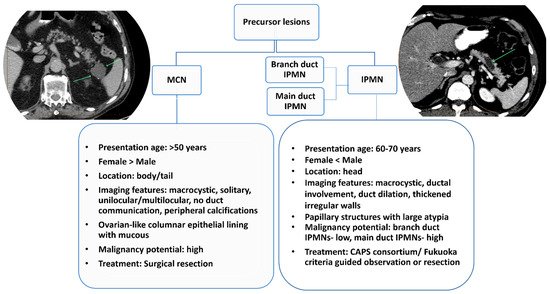
Figure 2. Characteristics of pancreatic cystic lesion types and their key differences. IPMN= intraductal papillary mucinous neoplasms; MCN= mucinous cystic neoplasms.
Mucinous cystic neoplasms, the least common macroscopic precursor lesion, differ from IPMNs in that they do not communicate with the ductal system, and they are almost always solitary. In addition, 98% of mucinous cystic neoplasms occur in perimenopausal women with a distinct proclivity to involve the distal pancreas (>90% occur in the tail region) and histologically characterised by a pathognomonic spindle cell ovarian-type stroma. Surgical resection is recommended in mucinous cystic neoplasms of greater than 4 cm, symptomatic tumours, and enhancing mural nodules. European evidence-based guidelines suggest that asymptomatic or smaller mucinous cystic neoplasms without suspicious features need long-term surveillance (every 6 months for 1st year and then annual) as long as they do not have surgical contraindications [
30]. The risk of malignant transformation of mucinous cystic neoplasms is lower than that of IPMN; approximately 16% of mucinous cystic neoplasms are associated with invasive malignancy. When malignancy develops, it is typically of the tubular carcinoma variant of PDAC, but lymph node metastasis is usually not seen.
5. Newly Onset Diabetes Mellitus
Although the association between diabetes mellitus and PDAC has been known since 1800s, the intricate relationship between the two conditions has yet to be fully understood. Diabetes appears to have a multidirectional association with PDAC. The onset of diabetes has been shown to precede occurrence of PDAC by a few years and resolve after the resection [
31]. The mechanism can be attributed to paraneoplastic phenomenon, leading to induced insulin resistance from pancreatic polypeptide deficiency. Although long standing type 2 diabetes is a modest risk factor (1.5–2-fold increased risk) for PDAC, newly onset diabetes may be a manifestation of PDAC [
32]. It has been suggested that up to 85% of pancreatic cancer patients have diabetes or hyperglycaemia which can manifest 2–3 years before the development of PDAC [
32]. This has been attributed to growth stimulation by endogenous hyperinsulinemia. Increased risks due to obesity and metabolic syndrome are also thought to arise from elevated insulin levels. For this newly identified high-risk group, there are no established guidelines or screening programs. Increasing epidemiological, clinical, and experimental evidence that newly onset diabetes is a clinical manifestation of asymptomatic PDAC provides hope for the early detection of PDAC in patients with diabetes.
6. Pancreatitis
Acute pancreatitis can be the initial clinical presentation of PDAC and can precede the diagnosis of PDAC by several weeks or months [
33]. Few studies have reported a detailed list of the aetiology of acute pancreatitis in their study cohort [
34,
35,
36,
37]. Estimated 1.7–3.6% of patients with acute pancreatitis were finally diagnosed with PDAC. The pooled average was 2.03% in 2945 patients in these studies [
33]. Duell et al. reported a nearly seven-fold increased relative risk for pancreatic cancer in individuals with a history of pancreatitis (adjusted odds ratio, 6.9; 95% CI, 3.4–14.1) [
38]. Another study reported that 1.45% of patients with acute pancreatitis developed PDAC within the 2-year period, and the incidence of PDAC reduced in the third year; further, age 40 years and older was an added risk factor for PDAC [
39]. Cases of carcinoma in situ were recently reported to be the cause of acute pancreatitis [
40]. Acute pancreatitis can be an indicator of PDAC, and patients with acute pancreatitis should be observed for 2 years using diagnostic imaging techniques [
33]. In chronic pancreatitis, >2-year observation showed that the relative risk for PDAC was 16.5 to 26.7, and the incidence ratios of PDAC occurrence were reported to be 1.1%, 1.8%, and 4.0% in 5-, 10- and 20-year observations, respectively [
41]. Hereditary pancreatitis is defined by the following: acute recurrent pancreatitis or chronic pancreatitis in two and more members of a family; an absence of a history of alcohol abuse in at least one patient; and pancreatitis in at least one brother or sister younger than 40 years. If the patient has a p.R122H or p.N291 mutation on PRSS1, the diagnosis of hereditary pancreatitis is confirmed, irrespective of the definition. In hereditary pancreatitis, cumulative lifetime risk of PDAC is 40% [
42]. The proportions of patients developing PDAC due to hereditary pancreatitis are 10%, 18.7%, and 53.5% in 50-, 60-, and 75-year-old patients, respectively [
42]. Familial history of pancreatitis should be considered when examining a patient with pancreatitis.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14102539