1. Obesity and Cell-Cycle Progression in Cancer
Obesity has become an emergent pandemic involving 1/3 of the population worldwide, a multifaceted disorder characterized by the overabundant accumulation of adipocytes—fat cells—which in turn may aggravate the course of different types of chronic diseases. In fact, more aggressive tumor profiles have been seen in overweight breast cancer patients, where adipocytes can secrete hormones, growth factors, and adipokines and release free fatty acids (FFA). The energy obtained from the β-oxidation of FFA can be used by adipocytes to accelerate tumor cell growth and cancer progression, stimulating the oncogenic signaling and leading to angiogenesis and malignant cell migration [
28,
29]. Similarly, ovarian cancer (OC) risk has also been correlated with elevated BMI and lipid levels. A significant 16–30% increased predisposition to OC has been identified in obese women, with a major risk assigned to specific histological subtypes—mostly endometrioid and mucinous carcinomas—and postmenopausal patients [
30]. This pathological condition is frequently associated with the overexpression of pro-inflammatory factors, cytokines, and adipokines, a process promoted by macrophage infiltration within the adipose tissue and able to exert tumor-promoting effects [
31]. Actually, the delivery of pro-inflammatory metabolites in the bloodstream may degenerate into hypothalamus deregulation, cause the loss of energy homeostasis, and bring to the disruption of crucial biological pathways, including those determining cell-cycle regulation. As a result, elevated levels of markers of inflammation such as tumor necrosis factor-alpha (TNF-α), interleukins (IL) 1—6-8, plasminogen activator inhibitor 1 (PAI1 or SERPINE1), and C-reactive protein (CRP) are also frequently found in obese patients.
The maintenance of energy and body weight balance are critical processes mainly led by the hypothalamic neurons, which constitute one of the most essential targets for adipokines such as leptin, a product of the obese gene (Ob). Altered levels of leptin and related hormones with a key role in food intake and appetite stimuli—such as ghrelin or insulin—are also generally present among overweight patients [
32].
1.1. Adipokines' Role in Cell-Cycle Progression
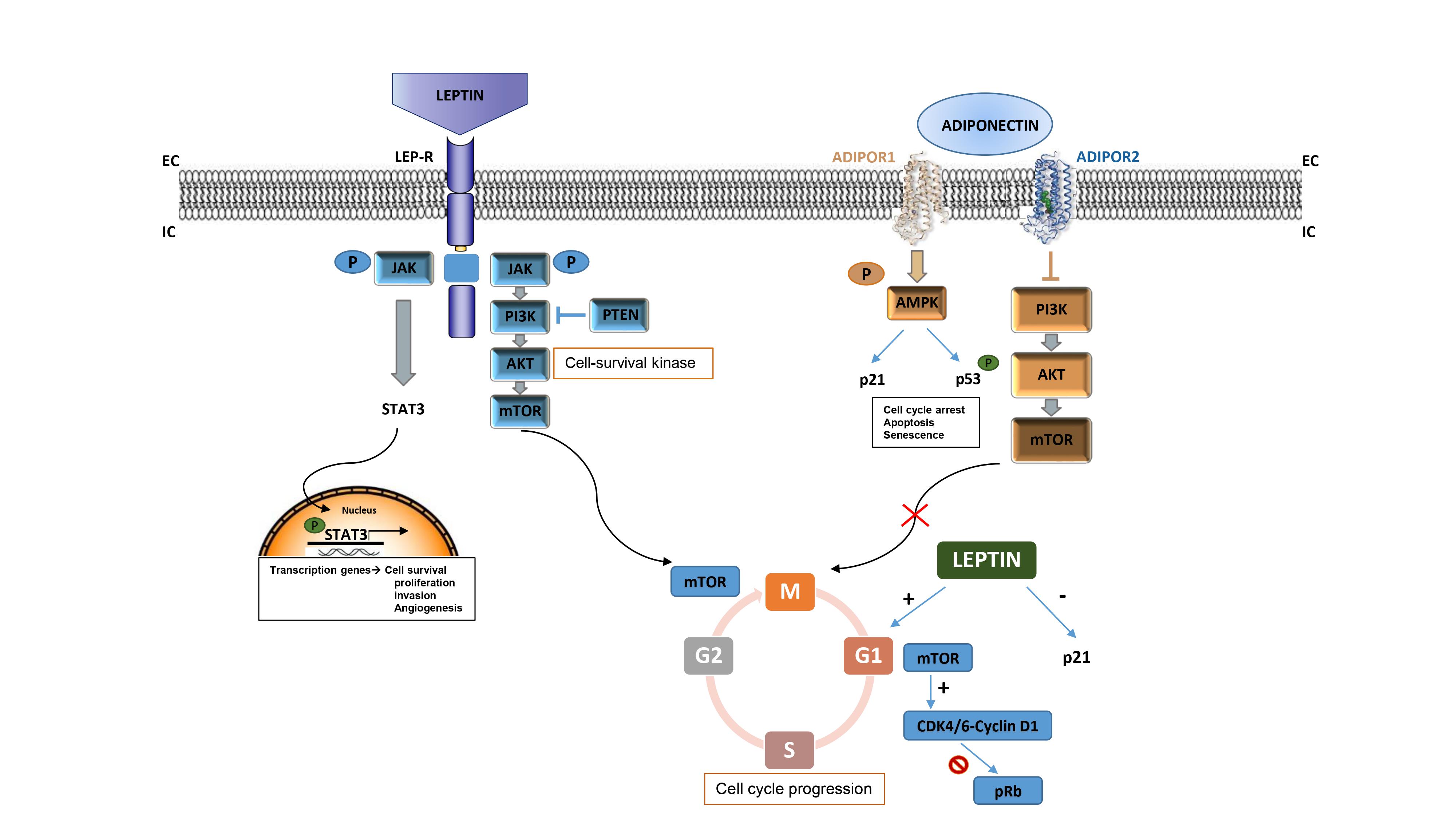
Adipokines are a group of cytokines secreted in the adipose tissue involved in the metabolic signaling in the brain, with an extensively demonstrated function in fostering cancer development. The most likely molecular mediators of inflammation from the adipose tissue itself are the adipokines leptin (discovered in 1994) and adiponectin (described for the first time in 1995)—critical for the maintenance of balanced bodyweight—and pro-inflammatory agents.
1.1.1. Leptin
A wide number of epidemiological studies are focusing on leptin hormone and its receptor (LepR) as good targets for the treatment approach of DIO, and have directly correlated the respective anorexigenic effects with the tumor cascade outgrowth. Leptin has been suggested to strongly take part in cancer onset and proliferation, by activating several growth signaling pathways such as PI3K/Akt, MAPKs (ERK1/2), and JAK/STAT3 [
32,
33], which drive cell-cycle progression acting on different target genes. These properties joined with the ability of leptin to promote angiogenesis, confer to this adipokine the main role as a growth factor for cancer cells [
34]. Indeed, some options presently under study are devoted to inhibiting the leptin cytokine cascade by using multiple approaches, including antibodies [
35], peptides [
36], and PPAR ligands [
37,
38]. Some of these leptin antagonists have been seen to efficiently arrest the leptin-induced cell-cycle progression at the S-phase in triple-negative breast cancer (TNBC) [
39,
40,
41].
Ptak et al. examined the relationship between OC development and leptin rates in obese women, identifying a key role of adipokine in stimulating cell cycle-related effectors. The research group connected the tumor proliferation role of leptin with a greater expression of cyclin D—indicative of a poorer prognosis—and cyclin A in an in vitro model; also identifying a downregulation of p21 [
33]. These results indicate leptin as a cell-cycle promoter, driving G1/S-phase transition in an OC model. Furthermore, an anti-apoptotic role of leptin was also observed in ovarian carcinogenesis through the blockade of caspase expression, which further stimulates cancer cell proliferation.
The expression levels of leptin constitute an interesting diagnostic tool that can be used to determine cancer risk, grade and type, stage, lymph node involvement, hormone receptors, and prognosis in breast [
42,
43,
44] and ovarian tumors [
45]. High leptin levels have been also correlated with lower chemosensitivity, and a common nexus between leptin and several mechanisms that come usually activated in breast tumors. Moreover, LEPR expression has been seen to be able to increase the cancer stem cell state in breast tumors, further promoting cell proliferation, stemness, and poorer survival [
50].
1.1.2. Adiponectin (APN)
In contrast to leptin’s effects on cancer, APN has been demonstrated to exert a protective role in the course of different malignancies, especially BC. APN is gaining interest in the management of obesity, where the extremely low levels of this hormone have been linked to insulin resistance, glucose metabolism, and thermogenesis processes. Interestingly, insulin promotes MCF-7 breast tumor cells proliferation and migration via PI3K activation [
51], and APN is able to downregulate the PI3K/Akt/mTOR cascade, resulting in an overall decrease in cancer cell viability, survival, and growth. Focusing on cancer signaling, this adipokine also activates AMPK, which induces cell-cycle arrest, apoptosis, and senescence via p21 activation and p53 phosphorylation. Moreover, APN upregulation also impedes STAT3 signal pathway activation, which in turn is unable to endorse angiogenesis and invasion and incapable of evading anticancer immunity, hence blocking tumor progression. The anti-inflammatory and pro-apoptotic effects of APN also involve the abolition of the NF-kB cascade by hampering NF-kB phosphorylation [
52,
53].
In an in vitro model of TNBC (MDA-MB-231 cell line), APN enhanced the overexpression of master genes that control cell-cycle progression, such as p53, and apoptosis (BAX, BCL2) [
54,
55]. This study also showed how the repression of the proto-oncogene MYC prevented cyclin D1 activation, consequently arresting the cell cycle at the G1/S-phase and hampering TNBC expansion. Nonetheless, the antiproliferative effects of APN in mammary cancers seem reliant on ERα expression, as an opposite role was observed in ERα + BC, where APN seems to promote cancer cell growth. On the opposite, low APN levels stimulate MAPK activation, which consequently phosphorylates SP1 and ER and enhances cyclin D1 expression, stimulating BC growth.
Despite the potential ERα-dependent effect of APN, patients with low leptin/APN ratios have shown a statistically longer cancer-specific survival for OC, which may show APN as a good candidate against DIO and derived metabolic diseases, including cancer [
56].
1.2. Additional Pro-Inflammatory Cytokines
The relationship between chronic inflammation, obesity, and several types of cancer has been extensively investigated, correlating the aggressiveness of tumor disease with higher levels of circulating inflammatory biomarkers such as cytokines [
57]. These functional proteins released by immune, stromal, and tumor cells affect cell proliferation via cell-cycle-regulatory proteins. As an example, the transforming growth factor-beta (TGF-β), is a key cytokine that in normal conditions induces tolerance and suppresses inflammation and in the early phases of tumorigenesis acts as a cytostatic tumor suppressive agent acting through p21 and p27 CDKI expression and inducing cancer cell apoptosis[
58,
59]. During tumor progression, however, TGF-β or its pathway is altered and decoupled from their tumor suppressor activity leading them to promote EMT and favoring a tumor immunosuppressive microenvironment that further enhances tumor invasiveness, as has been seen in HER2- BC [
59,
60].
Inflammatory cytokines such as IL-6, IL-21, IL-1β, and TNF-α diminish the cytotoxic capacity of immune CD8+ T cells to produce IFN-γ, which plays a main role in angiogenesis and MHC expression—tumor recognition. Higher values of these cytokines directly increase IL-17 production, activating angiogenesis and tumor growth [
61]. Indeed, risen expression of IL-1, IL-5, IL-6, IL-17, and NFκB were linked to aggressive phenotypes in BC patients and were correlated to a poorer prognosis and lower survival rates [
62,
63].
Interestingly, IL-6 has been demonstrated to switch on the JAK/STAT3 pathway and enhance EMT, which in turn promotes cancer proliferation, metastasis, and chemoresistance. Moreover, IL-6 is the main pro-inflammatory factor responsible for inducing the overexpression of tumor-related RAC1B, known to sustain tumor cell survival and promote escape from oncogene-induced senescence. Finally, increased levels of serum IL-6 have been correlated with poor prognosis, tumor size, and disease status [
64]. IL-17 can trigger the production of IL-6, which increases tumor cell migration and invasion, therefore contributing to tumor drug sensitivity and resistance to chemotherapy [
65].
IL-8 is also frequently found at high levels in BC, exerting inflammatory and angiogenic actions. In fact, a recently published study has shown that the pro-tumorigenic and metastatic effect of IL-8 passes by the activation of PI3K-Akt/MAPK and EMT signaling pathways leading to tumor cell migration[
66]. IL-4 instead upregulates adhesion molecules, inhibits cell proliferation and apoptosis, and mediates signal transduction in breast (MDA-MB-231) and ovarian tumors (SKOV-3), among others [
67]. Controversially, it has also been claimed that IL-4 possesses potent antitumor activity against various cancer types, including breast tumors, a reason by which additional research is needed prior to reaching a unique conclusion for this pro-inflammatory factor.
Interleukin-9 (IL-9) is a cytokine with pleiotropic functions that plays an important role in regulating tumor cell growth. IL-9 is increasingly produced by tumor-infiltrating T cells (TILs), as well as tumor cells themselves and a subset of Foxp3 expressing regulatory T cells (Tregs). FoxP3+ Treg cells are known to suppress antitumor immunity, suggesting that IL-9 derived from these cells might control immune responses [
68].
IL-10 is an immunosuppressive cytokine that can inhibit the ability of dendritic cells and macrophages to activate CD4 + T cells. IL-10 is frequently present at sites of chronic inflammation, promoting immunosuppression of humoral responses through the induction of isotype switching to IgG4. In a recent study, authors found a significant expression of IL-10 in tumor-infiltrating B-cells of TNBC patients, driving isotype switch to the IgG4 isotype in an IL-10 dependent manner [
69].
These observations suggest that IL-10 may play a role in directing antitumor immune escape. Moreover, both IgG4 and tumor IL-10 are associated with shorter recurrence-free survival (RFS) and overall survival (OS). In BC, IL-10 expression positively correlates with locally advanced disease and higher tumor grade and has been proposed as a good prognostic indicator of disease-free survival (DFS) [
70]. In melanoma, IL-10 expression by tumor cells is associated with melanoma progression [
71], while overexpression of serous IL-10 leads to an adverse survival in most cancer types [
72].
Pro-inflammatory stimuli have been seen to be able to raise pro-angiogenic factors in TNBC cells that physically interact with mesenchymal stem cells—MSCs—and stromal cells, accelerating the metastatic phenotype [
73]. Additionally, the Notch pathway, probably via CXCL8 cytokine release, has been demonstrated to promote the cell-to-cell interaction, affecting proliferation, differentiation, and death of cells—fostering TNBC spreading and invasion [
74]. In the same way, TNF-α has been seen to exert a tumor-promoting role in BC progression and induce metastasis, fostering tumor escape from immune system control [
75]. Nonetheless, some studies have recently addressed a controversial role of TNF-α, showing pro-apoptotic and anticarcinogenic functions towards different tumor types, which could also be dependent on TME or specific conditions such as the TNFR that controls the pathway or the ER/PR molecular BC type [
76,
77].
2. A Common Strategy in Cancer and DIO: Targeting Cell-Cycle Progression/CDKs
Several anti-obesity drugs are being tested for their potential interest as antitumoral agents, including lipid-lowering agents [
88]. For instance, the antihyperlipidemic agent orlistat has extensively proven to induce S-phase cell-cycle arrest and apoptosis in BC [
89]; whereas recently Harborg et al. carried out a cohort study that explored the link between the use of statins and the risk of developing BC, confirming an indirect relationship between them in postmenopausal early BC patients [
90]. Similarly, a noticeable 19% decrease in the OC demises was also noticed in a parallel study comparing mortality among statin users versus patients who never took statins before, with a major benefit assigned to Simvastatin [
91]. Furthermore, statin therapy not only did not entail a comparable toxic profile versus chemotherapy, but evidence also supports the ability of these antilipidemic drugs to promote apoptosis in malignant cells, reducing cancer progression and invasiveness [
92].
As one of the primordial purposes of oncosuppressors is to avoid cell-cycle progression by directly altering cyclin-dependent kinases expression, and CDK proteins are important players in cell-cycle modulation cascades, novel CDK inhibitors-based strategies have been proposed not only for the management of cancer but also for DIO and vice versa. The activity of these serine/threonine protein kinases is highly dependent on the activation of phase-specific cyclins, and the employment of CDKIs has emerged as an innovative strategy in tumor treatment [
25,
93,
94]. In the same way, multiple plant-derived biomolecules and by-products have shown CDK inhibitory functions raising interest as antitumoral agents [
95,
96,
97].
2.1. Food-Based Approaches in Cancer Therapy
Many anti-DIO strategies based on food intake time restrictions are being tested in vivo to better understand how specific nutritional deprivations affect different types of malignancies. These include several types of periodic fasting and intermittent food supply diets such as time-restricted feeding, short-restricted fasting, short-term starvation, alternate-day fasting, or fasting-mimicking diet (FMD). These fasting and dietary limitations are showing encouraging results in the management of obesity, notably impairing chronic disease burden and cancer onset [
98,
99].
Moreover, these strategies also represent a good and safe alternative that minimally affects non-tumoral cells, while selectively altering the survival chances of neoplastic cells, mainly by decreasing insulin and related factors, glucose, leptin, and cytokines [
100].
The ratio and specific type of macronutrients assumed can importantly change the course of the disease. Dietary patterns with a high content of animal-based proteins were correlated with a major risk of cancer demises compared with feeding habits mainly entailing vegetable-derived proteins [
101]. Following this thought, plant-based nutraceuticals are bringing attention as antitumor strategies, and micronutrients and phytochemicals of particular interest are undergoing clinical and preclinical trials in the cancer field.
To exemplify, more than 50 CTs have investigated the beneficial properties of broccoli-derived molecules (mainly sulforaphane and glucoraphanin) in cancer disease remission [
102,
103]. Nevertheless, only a few of them have brought into focus the molecular mechanisms involving the downregulation of cell cycle-related proteins such as cyclins and CDKs [
104], or the induction of CDKIs and correlated pathways involving signaling cascades such as the mammalian target of rapamycin (mTOR) [
105] or STAT 3[
106].
Indole-3-carbinol (I3C) represents an additional natural anticancer agent belonging to the same broccoli vegetable family (
Brassicaceae). It was found to block G1/S cell-cycle progression in breast and endometrial cancers, including MCF-7, BT20, and MDA-MB-231 cell lines [
121]. The effective reduction of cyclins D1, E, CDK-2, -4, and -6 and the increase of p21, p27, and p15 expression were also validated in response to I3C treatment [
97,
122].
Roscovitine constitutes another biological molecule employed in anti-DIO therapy with a key role in cell-cycle modulation. Specifically, its activity results in an accumulation of cells in the G2 phase on (ER-α)+ MCF-7 breast cancer cells, preventing them from entering the next cell cycle [
123].
Concomitantly to the inhibition of cell-cycle progression, roscovitine—later commercialized as Seliciclib
®, a first-generation CDKI—showed a remarkable ability to induce apoptosis via a p53-dependent pathway [
124]. Additionally, fangchinoline, an alkaloid isolated from the
Menispermaceae plant family, has been seen to impede G1/S cell cycle transition in MDA-MB-231 and MCF-7 breast cancer cells. The cell cycle blocking effects of fangchinoline alkaloid were further confirmed by a drop in the levels of cyclins D1, D3, and E; CDK-2, -4, and -6, as well as an increased expression of CDKIs p21 and p27 tumor suppressor proteins [
125].
The alkaloid Berberine has proved to have cytotoxic and antiproliferative actions in BC [
126] and OC cells [
127,
128], by targeting the Akt downstream pathway, whereas the flavonoid quercetin (
Quercus sp.) was able to stop the cell cycle at G1/S and G2/M checkpoints. Downregulation of Quercetin-3-methyl ether significantly prompted cell-cycle arrest at the G2-M phase in MDA-MB-231 and MCF-7 human BC cells, decreasing cell proliferation, invasion, and migration and inducing apoptosis [
129,
130]. A fall in CDK-2, -6, -7, and cyclins A, D1, and E were also confirmed [
131].
Curcumin is widely known to promote cell-cycle arrest at G1/S and G2/M phases and to stimulate the expression of tumor suppressor proteins p53, and the p21 and p27 endogenous CDKIs [
132,
133]. Preclinical studies indicated a beneficial effect of this
Curcuma longa-derived polyphenol in reducing severe skin side effects of radiotherapy in BC patients [
134]. Furthermore, a synergistic apoptotic action via PARP and p53 activation was seen in combined therapies of curcumin and citral extract, as well as an activation of the oxidative stress signaling via ROS production [
135].
2.2. Cyclin-Dependent Kinase Inhibitors as Anticancer Drugs
Flavopiridol was the first and most extensively studied CDK inhibitor entering human clinical trials to treat various cancer types, including breast, lung, and bladder [
138,
139]. This non-selective CDK inhibitor alkaloid initially proved to induce cell-cycle arrest in G0/G1 and an S-phase delay, showing a high specificity against the CDK1/cyclin B complex in BC [
140]. Even if the efficacy of flavopiridol in vivo has not been demonstrated to be sufficient to enable it to enter Phase III trials [
139] since the FDA approval of this CDKi as an orphan drug for acute myeloid leukemia in 2015, a larger set of molecules have entered clinical testing to evaluate their feasibility in the cancer treatment approach. Indeed, flavopiridol has opened a new window of opportunity for next-generation CDKIs, which means a higher drug specificity by the abolition of cyclin/CDK binding, which consequently impedes the protein complex-associated kinase activity and the subsequent cell-cycle progression. Among these new CDKIs, selective inhibitors of CDK4/6 are particularly gaining the major focus of interest [
141], whose activation is mainly dependent on cyclin D-type linkage.
Additional second-generation CDKIs include dinaciclib, a potent inhibitor that targets CDK1, CDK2, CDK5, and CDK9. Despite contrasting results arising, a Phase III randomized study revealed enthralling results in refractory leukemia patients in terms of efficacy, safety, and progression-free survival (PFS) [
142]. Moreover, in vitro tests guaranteed the major ability of dinaciclib to suppress Rb phosphorylation versus flavopiridol, subsequently validating a notable cell-cycle arrest in a huge number of malignant cell-based assays [
143]. CDK7 inhibitors are also emerging as anticancer therapeutic drugs by targeting diverse pathways, chiefly involving cell-cycle regulators such as CDK-activating kinase, that finally hinder the initiation of oncogenic transcription [
93,
144]. To date, four different CDK7i are under Phase II studies with encouraging outcomes in breast [
145] and ovarian cancers [
146,
147].
CDK-4 has been identified as a potential blocking target in diet-related anti-obesity treatment, as it promotes adipogenicity [
148]. The previously mentioned work of Iqbal and co-workers also described how lipid-enriched diets can induce pRb phosphorylation in the hypothalamus, which consequently inactivates the protein and promotes obesity in vivo. In fact, experiments carried out in mice treated with a first-generation CDKI—abemaciclib—have reported promising results in fat mass reduction and weight loss, and future assays are aimed to deduct which are the molecular mechanisms that may link the abrogation of CDK4/6 and the unphosphorylated form of pRb with the blockade of DIO in neurons [
22].
Interestingly, three different CDKIs 4/6 have recently been FDA approved for the treatment of lifelong aggressive and refractory HR+, Her2- BC therapy (palbociclib, PD0332991; ribociclib, LEE011; abemaciclib, LY835219) [
149,
150,
151]. Several biomolecular pathways induced during the obese condition have been demonstrated to be in common with cancer mechanisms of tumor evasion, prompting the study of CDK inhibitors for the management of obesity disease [
22]. With this scope, a common strategy linking FDA-authorized CDKIs, nutraceuticals, and dietary approaches could become a feasible tactic to handle overweight-related problems that may potentially favor cancer development.
Palbociclib (PD-0332991) was the first CDKI 4/6 demonstrating a substantial efficacy against breast cancer cells in combination with endocrine therapy in ER+ tumor models in vitro [
152]. From 2009 onwards, palbociclib has undergone successful studies in concomitance with hormonal therapies—HT—that led to its FDA approval in 2015 [
153], including selective estrogen receptor degraders (SERDs) [
154], aromatase inhibitors—AIs—[
155], and fulvestrant [
156]. Some CTs also show the activity of this CDK4/6 blocker as a single agent both in ovarian [
157] and in metastatic breast cancers [
158], illustrating a good drug side-effect profile.
Cytotoxicity was dose-dependent but showed some variability from one cell line to another; moreover, a direct correlation between p16 hypoexpression, high RB1 levels, and a significant response to palbociclib treatment was verified, both in vitro and in a clinical cohort of 263 OC patients. Inhibition of RB1 phosphorylation and promotion of G1 cell-cycle arrest and apoptosis further supported the promising use of palbociclib in OC, also in later clinical studies [
160].
Another noteworthy observation was carried out in TNBC models, a highly aggressive BC subtype characterized by the lack of expression of targetable receptors and a rapid tendency to metastasize to lungs, brain, and bones.
Abemaciclib exhibited the highest potency and best delivery efficiency among the three next-generation CDKIs, also showing effects on other kinases such as CDK9 and PIM1 [
162,
163]. Patnaik et al. (2016) performed preclinical studies in OC human xenografts and patients undergoing abemaciclib therapy [
164]. Promising results showed a good safety profile and clinical significance for this CDK 4/6 inhibitor, and a favorable and extended CA-125 response to treatment in advanced OC models. Novel studies concerning abemaciclib monotherapy have also been conducted in HR+/Her2- MBC patients who become refractory to endocrine therapy. Among these, the MONARCH-1 trial showed an overall response that accounted for 19.7% of total enrolled patients, whereas clinical benefit exceeded the 42% [
165], additionally confirming the antitumor activity and manageable toxicity profile of abemaciclib administered alone [
166].
In 2018, the MONALEESA-3 clinical trial outcomes prompted the FDA approval of ribociclib plus fulvestrant in postmenopausal HR+, Her2- advanced BC patients [
182]. Following studies of Iyengar and co-workers evaluated the effectiveness of selective CDK4/6 inhibitor ribociclib in different models of high-grade serous ovarian cancer both in vitro and in vivo and identified a pivotal and very selective dose-response activity against cancer cells viability [
186]. Cytotoxicity was even more evident upon ribociclib plus cisplatin association, showing a pronounced synergism in co-treatment therapies. The wide ability of CDK4/6 inhibitors to impede cell-cycle progression through the G0/G1 phase was confirmed, whereas the accumulation of cells at the G2/M phase suggested a potential role of ribociclib at this checkpoint as well. Interestingly, the addition of ribociclib also prevented cisplatin chemotherapy-surviving cells to progress over the G2/M cell-cycle phase. Combinations of ribociclib and letrozole also revealed promising results in early HR+ mammary tumors, identifying CDKIs as valuable alternatives to reduce relapses and side effects derived from ET [
184,
187].
So as the main mechanism underlying G1-targeted CDK4/6 inhibitors go through avoiding RB1 tumor suppressor phosphorylation and its subsequent inactivation, the effects of some agents such as palbociclib require the presence of a functional pRb protein to work properly [
188]. Cancers presenting deletions at the pRb protein level represent a treatment challenge, as the lack of a functional target makes these tumors resistant to CDKIs 4/6, making single-agent therapy ineffective [
189].
For this reason, CDK 4/6 inhibitors are presently experiencing multiple large, randomized clinical trials to test the prospective combined approaches with anti-estrogen and hormonal therapies [
153,
171,
190,
191]. Actually, therapies targeting ER such as tamoxifen, aromatase inhibitors, or fulvestrant that also affect cyclin D1 expression and promote G1 phase cell accumulation may potentiate the blocking function of a CDK4/6 inhibitor in cell-cycle progression [
153,
155,
173]. As a matter of fact, cyclin D1 has strongly exhibited a main role in the development of Her2-driven breast tumors [
192].
Intriguing early stage trials are also investigating possible CDKIs and PI3K inhibitors combinations in TNBC. Both palbociclib and ribociclib were administered together with taselisib/alpelisib—respectively—showing a greater synergistic response in terms of cell-cycle arrest and apoptosis compared to single-agent use [
193,
194].
There are several ongoing and completed Phase II/III studies testing dual CDK4/6 inhibitors against breast and ovarian carcinoma, further verifying the antitumor effectiveness of this group of drugs, some of which already manifesting superiority over ET in monotherapy. Additional trials combining palbociclib with other agents (e.g., capecitabine) did not exert comparable results in terms of clinical benefit, QoL, and safety profile [
195].
This entry is adapted from the peer-reviewed paper 10.3390/cancers14112709

