Pseudomonas aeruginosa is a common cause of healthcare-associated infections and chronic airway diseases in non-clinical settings. P. aeruginosa is intrinsically resistant to a variety of antimicrobials and has the ability to acquire resistance to others, causing increasingly recalcitrant infections and elevating public health concerns. We reviewed the literature on multidrug-resistant (MDR) P. aeruginosa isolated from humans (nosocomial and community-associated), animals, and the environment in Lebanon, a country that has been suffering from a surge in antimicrobial resistance (AMR). We identified 24 studies that described the epidemiology and antimicrobial susceptibility profiles of P. aeruginosa. Our analysis showed that the bacterium was predominant in lesions of patients on mechanical ventilation and in burn patients and those with diabetic foot infections and hematological malignancies. We also found that carbapenem resistance in P. aeruginosa isolates in Lebanon involved both enzymatic and non-enzymatic mechanisms but depended predominantly on VIM-2 production (40.7%). Additionally, MDR P. aeruginosa was detected in animals, where a recent study reported the emergence of carbapenemase-producing P. aeruginosa in livestock in Lebanon. Notably, no studies evaluated the contribution of MDR P. aeruginosa in the environment to human infections. Taken together, our findings highlight the need for AMR surveillance programs and a national action plan to combat resistance in Lebanon.
- Pseudomonas aeruginosa
- MDR
- carbapenemase
- Lebanon
- Antibiotic Resistance
- Infectious Diseases
1. Introduction
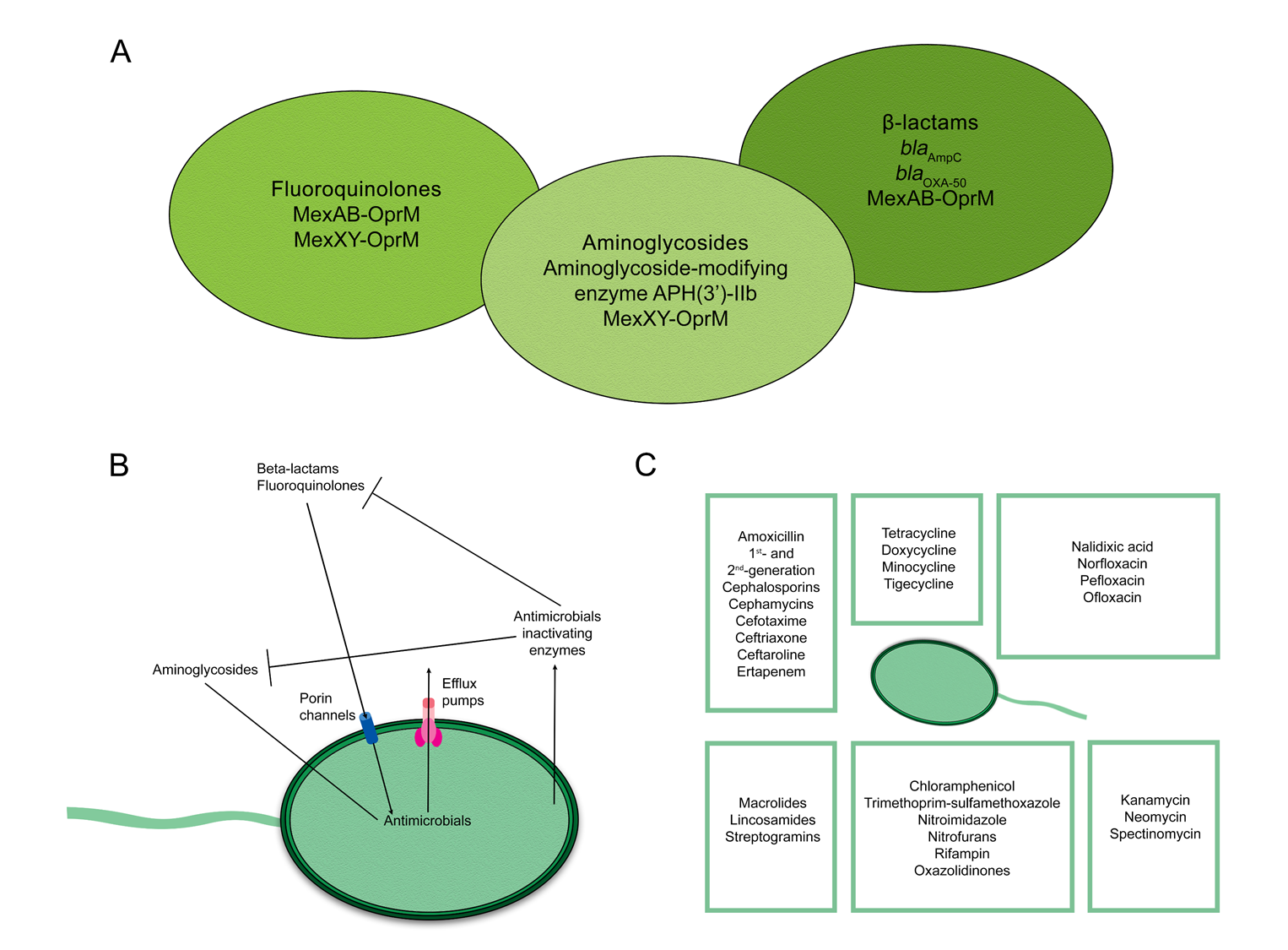
2. Mechanisms of Antimicrobial Resistance in Pseudomonas aeruginosa
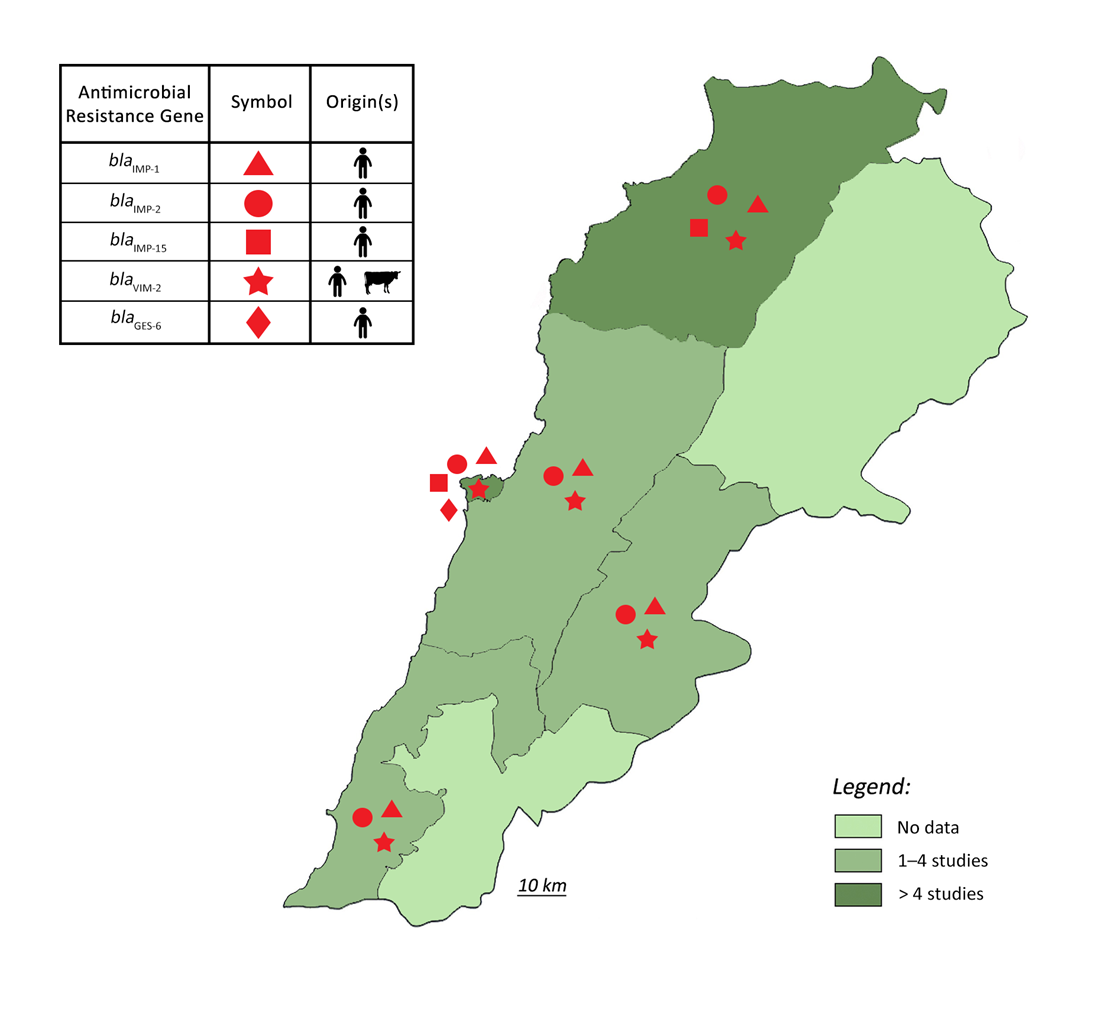
3. Epidemiology of Pseudomonas aeruginosa Resistance in Lebanon
4. Conclusions
5. References
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin. Microbiol. Rev. 2019, 32, e00031-19. [Google Scholar] [CrossRef] [PubMed]
- Filho, F.; Nascimento, A.P.; Costa, M.; Thiago, M.; Menezes, M.; Marisa, N.; Trindade dos Santos, M.; Carvalho-Assef, A.P.; da Silva, F. A systematic strategy to find potential therapeutic targets for Pseudomonas aeruginosa using integrated computational models. Front. Mol. Biosc. 2021, 8, 728129. [Google Scholar] [CrossRef] [PubMed]
- Abbara, A.; Rawson, T.M.; Karah, N.; El-Amin, W.; Hatcher, J.; Tajaldin, B.; Dar, O.; Dewachi, O.; Abu Sitta, G.; Uhlin, B.E.; et al. Antimicrobial resistance in the context of the Syrian conflict: Drivers before and after the onset of conflict and key recommendations. Int. J. Infect. Dis. 2018, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Halimeh, F.B.; Rafei, R.; Mallat, H.; Tom, J.E.; BouRaad, E.; Diene, S.D.; Jamal, S.; Al Atrouni, A.; Dabboussi, F.; et al. Investigation of an XDR-Acinetobacter baumannii ST2 outbreak in an intensive care unit of a Lebanese tertiary care hospital. Future Microbiol. 2020, 15, 1535–1542. [Google Scholar] [CrossRef]
- Hmede, Z.; Kassem, I.I. The Colistin Resistance Gene mcr-1 Is Prevalent in Commensal Escherichia coli Isolated from Preharvest Poultry in Lebanon. Antimicrob. Agents Chemother. 2018, 62, e01304-18. [Google Scholar] [CrossRef]
- Hmede, Z.; Sulaiman, A.A.A.; Jaafar, H.; Kassem, I.I. Emergence of plasmid-borne colistin resistance gene mcr-1 in multidrug-resistant Escherichia coli isolated from irrigation water in Lebanon. Int. J. Antimicrob. Agents 2019, 54, 102–104. [Google Scholar] [CrossRef]
- Al-Omari, S.; Al Mir, H.; Wrayde, S.; Merhabi, S.; Dhaybi, I.; Jamal, S.; Chahine, M.; Bayaa, R.; Tourba, F.; Tantawi, H.; et al. First Lebanese Antibiotic Awareness Week campaign: Knowledge, attitudes and practices towards antibiotics. J. Hosp. Infect. 2019, 101, 475–479. [Google Scholar] [CrossRef]
- Samer, S.; Ghaddar, A.; Hamam, B.; Sheet, I. Antibiotic use and resistance: An unprecedented assessment of university students’ knowledge, attitude and practices (KAP) in Lebanon. BMC Public Health 2020, 20, 535. [Google Scholar]
- Osman, M.; Kasir, D.; Kassem, I.I.; Hamze, M. Shortage of appropriate diagnostics for antimicrobial resistance in Lebanese clinical settings: A crisis amplified by COVID-19 and economic collapse. J. Glob. Antimicrob. Resist. 2021, 27, 72–74. [Google Scholar] [CrossRef]
- ESCWA. Escwa Warns: More Than Half of Lebanon’s Population Trapped in Poverty 2020. Available online: https://www.unescwa.org/news/lebanon-population-trapped-poverty (accessed on 6 May 2022).
- Kassem, I.I.; Osman, M. A brewing storm: The impact of economic collapse on the access to antimicrobials in Lebanon. J. Glob. Antimicrob. Resist. 2022; ahead of print. [Google Scholar] [CrossRef]
- Dadouch, S.; Durgham, N. Lebanon was Famed for Its Medical Care. Now, Doctors and Nurses are Fleeing in Droves. The Washington Post 2021. Available online: https://www.washingtonpost.com/world/middle_east/lebanon-crisis-healthcare-doctors-nurses/2021/11/12/6bf79674-3e33-11ec-bd6f-da376f47304e_story.html (accessed on 6 May 2022).
- Dagher, L.A.; Hassan, J.; Kharroubi, S.; Jaafar, H.; Kassem, I.I. Nationwide Assessment of Water Quality in Rivers across Lebanon by Quantifying Fecal Indicators Densities and Profiling Antibiotic Resistance of Escherichia coli. Antibiotics 2021, 10, 883. [Google Scholar] [CrossRef] [PubMed]
- Hassan, J.; Zein Eddine, R.; Mann, D.; Li, S.; Deng, X.; Saoud, I.P.; Kassem, I.I. The mobile colistin resistance gene, mcr-1.1, is carried on Incx4 plasmids in multidrug resistant E. coli isolated from rainbow trout aquaculture. Microorganisms 2020, 8, 1636. [Google Scholar] [CrossRef] [PubMed]
- Sourenian, T.; Mann, D.; Li, S.; Deng, X.; Jaafar, H.; Kassem, I.I. Dissemination of multidrug-resistant Escherichia coli harboring the mobile colistin resistance gene mcr-1.1 on transmissible plasmids in the Mediterranean Sea. J. Glob. Antimicrob. Resist. 2020, 22, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Al-Mir, H.; Osman, M.; Drapeau, A.; Hamze, M.; Madec, J.-Y.; Haenni, M. Spread of ESC-, carbapenem- and colistin-resistant Escherichia coli clones and plasmids within and between food workers in Lebanon. J. Antimicrob. Chemother. 2021, 76, 3135–3143. [Google Scholar] [CrossRef]
- Al-Mir, H.; Osman, M.; Drapeau, A.; Hamze, M.; Madec, J.-Y.; Haenni, M. WGS Analysis of Clonal and Plasmidic Epidemiology of Colistin-Resistance Mediated by mcr Genes in the Poultry Sector in Lebanon. Front. Microbiol. 2021, 12, 624194. [Google Scholar] [CrossRef]
- Osman, M.; Al Mir, H.; Rafei, R.; Dabboussi, F.; Madec, J.-Y.; Haenni, M.; Hamze, M. Epidemiology of antimicrobial resistance in Lebanese extra-hospital settings: An overview. J. Glob. Antimicrob. Resist. 2019, 17, 123–129. [Google Scholar] [CrossRef]
- Kassem, I.I.; Hijazi, M.A.; Saab, R. On a collision course: The availability and use of colistin-containing drugs in human therapeutics and food-animal farming in Lebanon. J. Glob. Antimicrob. Resist. 2019, 16, 162–164. [Google Scholar] [CrossRef]
- Kassem, I.I.; Nasser, N.A.; Salibi, J. Prevalence and Loads of Fecal Pollution Indicators and the Antibiotic Resistance Phenotypes of Escherichia coli in Raw Minced Beef in Lebanon. Foods 2020, 9, 1543. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Hirsch, E.B.; Brigman, H.V.; Zucchi, P.C.; Chen, A.; Anderson, J.C.; Eliopoulos, G.M.; Cheung, N.; Gilbertsen, A.; Hunter, R.C.; Emery, C.L.; et al. Ceftolozane-tazobactam and ceftazidime-avibactam activity against b-lactam-resistant Pseudomonas aeruginosa and extended-spectrum b-lactamase-producing enterobacterales clinical isolates from U.S. medical centres. J. Glob. Antimicrob. Resist. 2020, 22, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Cultrera, R.; Libanore, M.; Barozzi, A.; D’Anchera, E.; Romanini, L.; Fabbian, F.; De Motoli, F.; Quarta, B.; Stefanati, A.; Bolognesi, N.; et al. Ceftolozane/Tazobactam and Ceftazidime/Avibactam for Multidrug-Resistant Gram-Negative Infections in Immunocompetent Patients: A Single-Center Retrospective Study. Antibiotics 2020, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, R.; Hijazi, K.; Joshi, C.; Aitken, E.; Oggioni, M.R.; Gould, I.M. Phylogenetic analysis of resistance to ceftazidime/avibactam, ceftolozane/tazobactam and carbapenems in piperacillin/tazobactam-resistant Pseudomonas aeruginosa from cystic fibrosis patients. Int. J. Antimicrob. Agents 2019, 53, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updat. 2010, 13, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, S.; Li, X.; He, X.; Jian, L. Development of in vitro resistance to fluoroquinolones in Pseudomonas aeruginosa. Antimicrob. Resist. Infect. Control. 2020, 9, 124. [Google Scholar] [CrossRef]
- Walters, M.S.; Grass, J.E.; Bulens, S.N.; Hancock, E.B.; Phipps, E.C.; Muleta, D.; Mounsey, J.; Kainer, M.A.; Concannon, C.; Dumyati, G.; et al. Carbapenem-resistant Pseudomonas aeruginosa at US emerging infections program sites, 2015. Emerg. Infect. Dis. 2019, 25, 1281–1288. [Google Scholar] [CrossRef]
- Hamel, M.; Rolain, J.-M.; Baron, S. The History of Colistin Resistance Mechanisms in Bacteria: Progress and Challenges. Microorganisms 2021, 9, 442. [Google Scholar] [CrossRef]
- Fernández, L.; Álvarez-Ortega, C.; Wiegand, I.; Olivares, J.; Kocíncová, D.; Lam, J.S.; Martínez, J.L.; Hancock, R.E.W. Characterization of the Polymyxin B Resistome of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 110–119. [Google Scholar] [CrossRef]
- Pathak, A.; Singh, S.; Kumar, A.; Prasad, K. Emergence of chromosome borne colistin resistance gene, mcr-1 in clinical isolates of Pseudomonas aeruginosa. Int. J. Infect. Dis. 2020, 101, 22. [Google Scholar] [CrossRef]
- El-Baky, R.M.A.; Masoud, S.M.; Mohamed, D.S.; Waly, N.G.; Shafik, E.A.; Mohareb, D.A.; Elkady, A.; Elbadr, M.M.; Hetta, H.F. Prevalence and Some Possible Mechanisms of Colistin Resistance Among Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa. Infect. Drug Resist. 2020, 13, 323–332. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Chandler, C.E.; Leung, L.M.; McElheny, C.L.; Mettus, R.T.; Shanks, R.M.Q.; Liu, J.-H.; Goodlett, D.R.; Ernst, R.K.; Doi, Y. Structural Modification of Lipopolysaccharide Conferred by mcr-1 in Gram-Negative ESKAPE Pathogens. Antimicrob. Agents Chemother. 2017, 61, e00580-17. [Google Scholar] [CrossRef] [PubMed]
- Snesrud, E.; Maybank, R.; Kwak, Y.I.; Jones, A.R.; Hinkle, M.K.; McGann, P. Chromosomally Encoded mcr-5 in Colistin-Nonsusceptible Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2018, 62, e00679-18. [Google Scholar] [CrossRef] [PubMed]
- Abbott, I.J.; Van Gorp, E.; Wijma, R.A.; Dekker, J.; Croughs, P.D.; Meletiadis, J.; Mouton, J.W.; Peleg, A.Y. Efficacy of single and multiple oral doses of fosfomycin against Pseudomonas aeruginosa urinary tract infections in a dynamic in vitro bladder infection model. J. Antimicrob. Chemother. 2020, 75, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Mustapha, M.M.; Tomich, A.D.; Callaghan, J.D.; McElheny, C.L.; Mettus, R.T.; Shanks, R.M.Q.; Sluis-Cremer, N.; Doi, Y. Widespread Fosfomycin Resistance in Gram-Negative Bacteria Attributable to the Chromosomal fosA Gene. MBio 2017, 8, e00749-17. [Google Scholar] [CrossRef]
- Díez-Aguilar, M.; Morosini, M.I.; Tedim, A.P.; Rodríguez, I.; Aktaş, Z.; Cantón, R. Antimicrobial Activity of Fosfomycin-Tobramycin Combination against Pseudomonas aeruginosa Isolates Assessed by Time-Kill Assays and Mutant Prevention Concentrations. Antimicrob. Agents Chemother. 2015, 59, 6039–6045. [Google Scholar] [CrossRef]
- Al Bayssari, C.; Dabboussi, F.; Hamze, M.; Rolain, J.-M. Emergence of carbapenemase-producing Pseudomonas aeruginosa and Acinetobacter baumannii in livestock animals in Lebanon. J. Antimicrob. Chemother. 2015, 70, 950–951. [Google Scholar] [CrossRef]
- Moghnieh, R.; Araj, G.F.; Awad, L.; Daoud, Z.; Mokhbat, J.E.; Jisr, T.; Abdallah, D.; Azar, N.; Irani-Hakimeh, N.; Balkis, M.M.; et al. A compilation of antimicrobial susceptibility data from a network of 13 Lebanese hospitals reflecting the national situation during 2015–2016. Antimicrob. Resist. Infect. Control. 2019, 8, 41. [Google Scholar] [CrossRef]
- Chamoun, K.; Farah, M.; Araj, G.; Daoud, Z.; Moghnieh, R.; Salameh, P.; Saade, D.; Mokhbat, J.; Abboud, E.; Hamze, M.; et al. Surveillance of antimicrobial resistance in Lebanese hospitals: Retrospective nationwide compiled data. Int. J. Infect. Dis. 2016, 46, 64–70. [Google Scholar] [CrossRef]
- Halat, D.H.; Moubareck, C.A.; Sarkis, D.K. Heterogeneity of Carbapenem Resistance Mechanisms Among Gram-Negative Pathogens in Lebanon: Results of the First Cross-Sectional Countrywide Study. Microb. Drug Resist. 2017, 23, 733–743. [Google Scholar] [CrossRef]
- Shaar, T.; Al-Hajjar, R. Antimicrobial susceptibility patterns of bacteria at the Makassed General Hospital in Lebanon. Int. J. Antimicrob. Agents 2000, 14, 161–164. [Google Scholar] [CrossRef]
- Araj, G.F.; Uwaydah, M.M.; Alami, S.Y. Antimicrobial susceptibility patterns of bacterial isolates at the american university medical center in Lebanon. Diagn. Microbiol. Infect. Dis. 1994, 20, 151–158. [Google Scholar] [CrossRef]
- Araj, G.F.; Avedissian, A.Z.; Ayyash, N.S.; Bey, H.A.; El Asmar, R.G.; Hammoud, R.Z.; Itani, L.Y.; Malak, M.R.; Sabai, S.A. A reflection on bacterial resistance to antimicrobial agents at a major tertiary care center in Lebanon over a decade. J. Med. Liban. 2012, 60, 125–135. [Google Scholar] [PubMed]
- Kanafani, Z.A.; Kara, L.; Hayek, S.; Kanj, S.S. Ventilator-associated pneumonia at a tertiary-care center in a ceveloping country: Incidence, microbiology, and susceptibility patterns of isolated microorganisms. Infect. Control. Hosp. Epidemiol. 2003, 24, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Matar, G.M.; Chaar, M.H.; Araj, G.F.; Srour, Z.; Jamaleddine, G.; Hadi, U. Detection of a highly prevalent and potentially virulent strain of Pseudomonas aeruginosa from nosocomial infections in a medical center. BMC Microbiol. 2005, 5, 29. [Google Scholar] [CrossRef]
- Hamouche, E.; Sarkis, D.K. Evolution of susceptibility to antibiotics of Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumanii, in a university hospital center of Beirut between 2005 and 2009. Pathol. Biol. 2012, 60, e15–e20. [Google Scholar] [CrossRef]
- Al-Hajje, A.; Ezedine, M.; Hammoud, H.; Awada, S.; Rachidi, S.; Zein, S.; Salameh, P. Current status of nosocomial infections in the Lebanese Hospital Center, Beirut. East. Mediterr. Health J. 2012, 18, 495–500. [Google Scholar] [CrossRef]
- Jouhar, L.; Jaafar, R.F.; Nasreddine, R.; Itani, O.; Haddad, F.; Rizk, N.; Hoballah, J.J. Microbiological profile and antimicrobial resistance among diabetic foot infections in Lebanon. Int. Wound J. 2020, 17, 1764–1773. [Google Scholar] [CrossRef]
- Hammoudi, D.; Moubareck, C.A.; Kanso, A.; Nordmann, P.; Sarkis, D.K. Surveillance of carbapenem non-susceptible Gram negative strains and characterization of carbapenemases of classes A, B, and D in a Lebanese hospital. J. Med. Liban. 2015, 63, 66–73. [Google Scholar] [CrossRef]
- Bourgi, J.; Said, J.M.; Yaakoub, C.; Atallah, B.; Al Akkary, N.; Sleiman, Z.; Ghanimé, G. Bacterial infection profile and predictors among patients Aadmitted to a burn care center: A retrospective study. Burns 2020, 46, 1968–1976. [Google Scholar] [CrossRef]
- Yaghi, J.; Fattouh, N.; Akkawi, C.; El Chamy, L.; Maroun, R.G.; Khalil, G. Unusually High Prevalence of Cosecretion of Ambler Class A and B Carbapenemases and Nonenzymatic Mechanisms in Multidrug-Resistant Clinical Isolates of Pseudomonas aeruginosa in Lebanon. Microb. Drug Resist. 2020, 26, 150–159. [Google Scholar] [CrossRef]
- Dagher, T.N.; Al-Bayssari, C.; Diene, S.; Azar, E.; Rolain, J.-M. Emergence of plasmid-encoded VIM-2–producing Pseudomonas aeruginosa isolated from clinical samples in Lebanon. New Microbes New Infect. 2019, 29, 100521. [Google Scholar] [CrossRef] [PubMed]
- Hamze, M.; Dabboussi, F.; Izard, D. A 4-year study of Pseudomonas aeruginosa susceptibility to antibiotics (1998–2001) in northern Lebanon. Med. Mal. Infect. 2004, 34, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Al Bayssari, C.; Diene, S.M.; Loucif, L.; Gupta, S.K.; Dabboussi, F.; Mallat, H.; Hamze, M.; Rolain, J.-M. Emergence of VIM-2 and IMP-15 Carbapenemases and Inactivation of oprD Gene in Carbapenem-Resistant Pseudomonas aeruginosa Clinical Isolates from Lebanon. Antimicrob. Agents Chemother. 2014, 58, 4966–4970. [Google Scholar] [CrossRef] [PubMed]
- Hamze, M.; Osman, M.; Mallat, H.; Achkar, M. Prevalence and antibiotic susceptibility of ear pathogens isolated from patients in Tripoli, north of Lebanon. Int. Arab. J. Antimicrob. Agents 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Hamze, M.; Mallat, H.; Dabboussi, F.; Achkar, M. Antibiotic susceptibility, serotyping and determination of minimum inhibitory concentrations of imipenem and meropenem in relation to 88 Strains of Pseudomonas aeruginosa isolated in northern Lebanon. Int. Arab. J. Antimicrob. Agents. 2012, 2, 1–6. [Google Scholar]
- Osman, M.; Mallat, H.; Hamze, M.; Bou Raad, E. Prevalence and antibiotic susceptibility patterns of bacteria causing urinary uract infections in Youssef hospital center: First report from akkar governorate, north Lebanon. Int. Arab. J. Antimicrob. Agents 2017, 7, 2–4. [Google Scholar] [CrossRef]
- Hamze, M.; Osman, M.; Mallat, H.; Nasr, S.; BouRaad, E.; Achkar, M. Epidemiology and antibiotic susceptibility patterns of carbapenem-resistant Gram-negative bacteria isolated from two tertiary care hospitals in north Lebanon. Int. Arab. J. Antimicrob. Agents 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Al-Bayssari, C.; Dagher, T.N.; El Hamoui, S.; Fenianos, F.; Makdissy, N.; Rolain, J.-M.; Nasreddine, N. Carbapenem and colistin-resistant bacteria in North Lebanon: Coexistence of MCR-1 and NDM-4 genes in Escherichia coli. J. Infect. Dev. Ctries. 2021, 15, 934-342. [Google Scholar] [CrossRef]
- Fares, Y.; El-Zaatari, M.; Fares, J.; Bedrosian, N.; Yared, N. Trauma-related infections due to cluster munitions. J. Infect. Public Health 2013, 6, 482–486. [Google Scholar] [CrossRef]
- van Duin, D.; Bonomo, R.A. Ceftazidime/avibactam and ceftolozane/tazobactam: Second-generation b-lactam/b-lactamase inhibitor combinations. Clin. Infect. Dis. 2016, 63, 234–241. [Google Scholar] [CrossRef]
- Araj, G.F.; Berjawi, D.M.; Musharrafieh, U.; El Beayni, N.K. Activity of ceftolozane/tazobactam against commonly encountered antimicrobial resistant Gram-negative bacteria in Lebanon. J. Infect. Dev. Ctries. 2020, 14, 559–564. [Google Scholar] [CrossRef] [PubMed]
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics11050687
