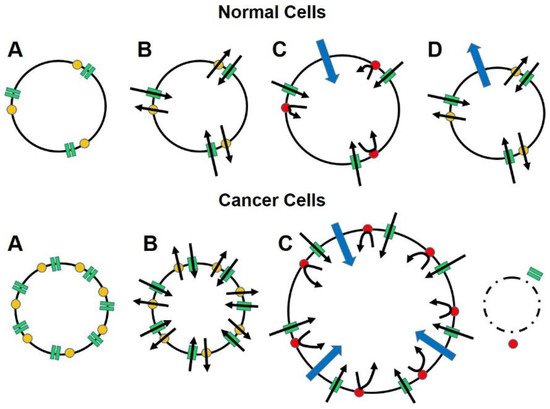
In the absence of a preventive vaccine, we offer for consideration a novel and fundamentally different approach, both in principle and design, for treating advanced-stage carcinoma that exploits a basic biological mechanism for survival. The process, called “targeted osmotic lysis (TOL)”, takes advantage of the interdependent, sodium channel/sodium pump alliance that is present in the cells of all animals and is essential for cell communication and survival because of its role in maintaining membrane potential and cellular homeostasis [
38,
39]. TOL technology is based on the observation that many epithelially-derived cancers over-express voltage-gated sodium channels (VGSCs) and Na
+, K
+-ATPase, a feature that confers an enhanced ability to invade normal tissue and to metastasize and is found to be exceedingly prominent in advanced disease, and that the expression of VGSCs in the cancer cells is directly related to the level of malignancy [
38,
40,
41,
42]. Although blocking the expression or impeding the function of VGSCs by pharmacologic means has been shown to slow tumor growth and reduces metastasis, these agents leave the original tumor intact and are associated with variable adverse effects imposed on cells that normally express VGSCs [
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58]. Unlike most targeted technologies that selectively deliver lethal therapies to targeted cells by identifying cellular markers unique to the specific cancer cells, TOL enhances, rather than impedes, VGSC marker functionality, thereby greatly increasing the influx of sodium while simultaneously preventing extrusion of these ions by blocking the sodium pumping mechanism with a cardiac glycoside (
Figure 1). Because water passively follows sodium by osmosis and possibly through aquaporins [
59], the cells swell beyond their capacity to comply, resulting in cell lysis. Normal cells, even highly-expressing excitable cells (e.g., nerve and muscle) are spared from damage because sodium channel expression in normal tissues is significantly less than that found in most advanced carcinomas. Less sodium, and consequently less water, enters normal cells precluding significant cell swelling and lysis. Unlike destructive therapies that deliver irreversible, lethal agents that destroy all recognized cells, whether malignant or normal, TOL only lyses highly malignant cells that are set apart from normal by the up to 50× greater expression of VGSCs than normal cells [
60]. Although TOL affects all cells during active treatment, the Na
+, K
+-ATPase blockade is reversible, thus allowing normal cells that do not take on enough water for lysis to return to normal when the cardiac glycoside is released from the receptor and metabolized without producing significant morbidity in the patient.