The discovery of ferrocene is often associated with the rapid growth of organometallic chemistry. Dendrimers are highly branched macromolecules that can be functionalized at will at all levels of their structure. The functionalization of dendrimers with ferrocene derivatives can be carried out easily as terminal functions on the surface, but also at the core, or at one or several layers inside the structure. Depending on the desired location of the ferrocenes in the structure of phosphorhydrazone dendrimers, the ferrocenes should be functionalized differently. For the grafting to the surface, the ferrocene should bear a phenol group, suitable to react in substitution reactions with the P(S)Cl2 terminal groups of the dendrimers. To be used as core, the ferrocene should have two aldehyde functions, from which the synthesis of the dendrimer will be carried out. To be introduced in the branches, at all layers or within a single layer, the ferrocene should replace hydroxybenzaldehyde; thus, it should bear both a phenol and an aldehyde.
- ferrocene
- dendrimer
- catalysis
- electrochemistry
1. Introduction
Thousands of publications concern ferrocene derivatives, and their discovery represented a turning point in organometallic chemistry, as the leading member of the metallocene family. Ferrocene consists of two cyclopentadienyl rings bound on opposite sides of a central Fe2+ atom. It has been shown to have excellent thermal and photochemical stability, and to undergo a facile and reversible one-electron oxidation to ferrocenium cation. The functionalization of one or both cyclopentadienyl units has led to their grafting to numerous types of molecules, and to polymers and to dendrimers. Dendrimers are a very special class of polymers, constituted of repetitive branching units but not synthesized by polymerization reactions. Their step-by-step synthesis ensures a perfectly defined and highly reproducible structure [1]. Among the diverse types of dendrimers, phosphorhydrazone dendrimers, which possess one phosphorus atom at each branching point [2], occupy a special place among “inorganic” dendrimers [3] thanks to their easy and versatile functionalization, in particular on their periphery [4], and to the numerous properties that have been already demonstrated with them. Figure 1 displays the chemical structure of phosphorhydrazone dendrimers of generation 2, built either from P(S)Cl3 (1-G2) or cyclotriphosphazene N3P3Cl6 (2-G2) [5].
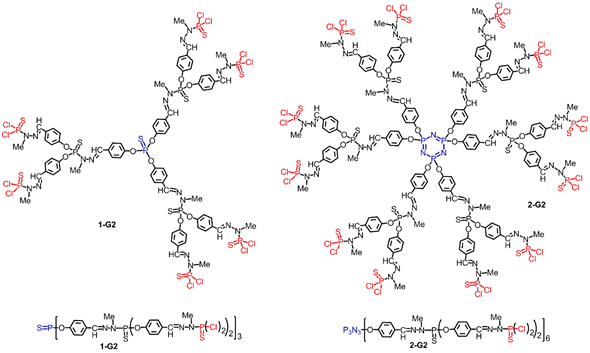
Figure 1. Structure of second generation phosphorhydrazone dendrimers. Left: built from P(S)Cl3 as core; right: built from N3P3Cl6 as core. Upper part: full structure; lower part: linear structures with parentheses to draw the same dendrimers.
Depending on the desired location of the ferrocenes in the structure of phosphorhydrazone dendrimers, the ferrocenes should be functionalized differently.
2. Ferrocenes on the Periphery of Dendrimers
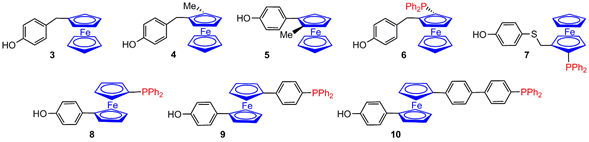
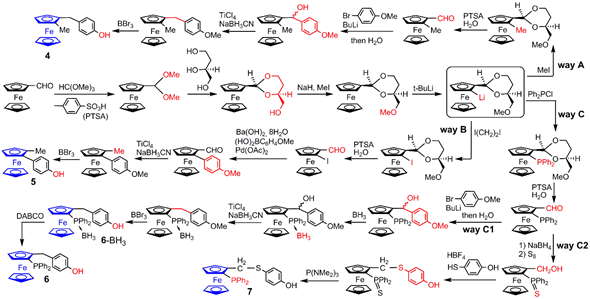
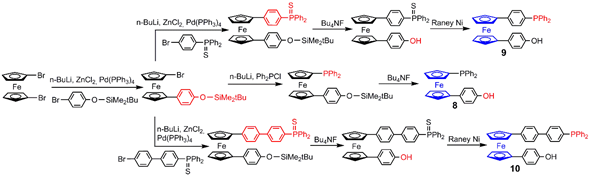
3. Ferrocene at the Core of Dendrimers
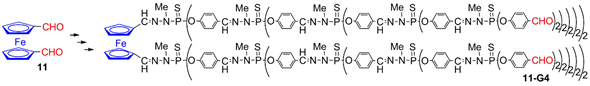
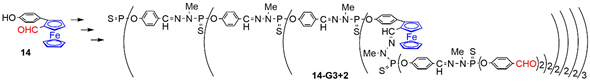
4. Ferrocenes as Branches of Dendrimers at one or Several Layers
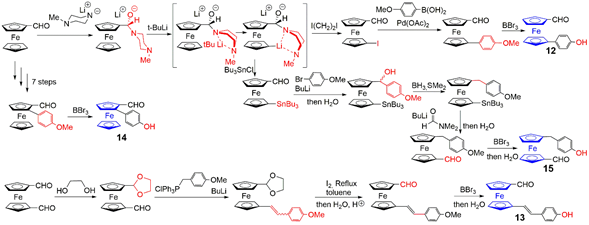
4.1. Ferrocenes at all Layers of Dendrimers
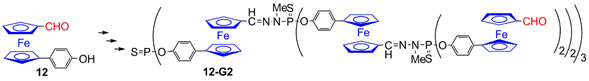
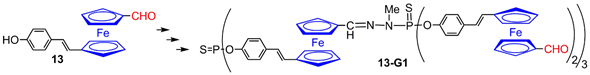
4.2. Ferrocenes at a Single Layer Inside Dendrimers
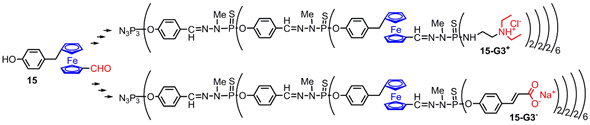
This entry is adapted from the peer-reviewed paper 10.3390/molecules25030447
References
- Caminade, A.-M.; Turrin, C.-O.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B.. Dendrimers: Towards Catalytic, Material and Biomedical Uses; Caminade, A.-M.; Turrin, C.-O.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B.;, Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2011; pp. 1–538.
- Launay, N.; Caminade, A.M.; Lahana, R.; Majoral, J.P.; A general synthetic strategy for neutral phosphorus-containing dendrimers. Angew. Chem.-Int. Ed. Engl. 1994, 33, 1589–1592, .
- Caminade, A.M.; Inorganic dendrimers: Recent advances for catalysis, nanomaterials, and nanomedicine. Chem. Soc. Rev. 2016, 45, 5174–5186, .
- Launay, N.; Slany, M.; Caminade, A.M.; Majoral, J.P.; Phosphorus-containing dendrimers. Easy access to new multi-difunctionalized macromolecules. J. Org. Chem. 1996, 61, 3799–3805, .
- Caminade, A.-M.; Hameau, A.; Majoral, J.-P.; The specific functionalization of cyclotriphosphazene for the synthesis of smart dendrimers. Dalton Trans. 2016, 45, 1810–1822, .
- Iftime, G.; Moreau-Bossuet, C.; Manoury, E.; Balavoine, G.G.A.; Selective functionalization of the 1′-position of ferrocenecarbaldehyde. Chem. Commun. 1996, , , .
- Chiffre, J.; Averseng, F.; Balavoine, G.G.A.; Daran, J.C.; Iftime, G.; Lacroix, P.G.; Manoury, E.; Nakatani, K.; A Novel and Perfectly Aligned Crystal of A Ferrocenyl Chromophore displaying High Quadratic Non Linear Optical Bulk Efficiency. Eur. J. Inorg. Chem. 2001, , , .
- Routaboul, L., Chiffre, J.; Balavoine, G.G.A.; Daran, J.C.; Manoury, E.; Highly efficient reduction of ferrocenyl derivatives by borane. J. Organomet. Chem. 2001, 637–639, 364–371, .
- Routaboul, L., Vincendeau, S.; Daran, J.C.; Manoury, E.; New ferrocenyl P,S and S,S ligands for asymmetric catalysis. . Tetrahedron Asymmetry 2005, 16, 2685–2690, .
- Turrin, C.O.; Chiffre, J.; de Montauzon, D.; Daran, J.C.; Caminade, A.M.; Manoury, E.; Balavoine, G.; Majoral, J.P.; Phosphorus-containing dendrimers with ferrocenyl units at the core, within the branches, and on the periphery. Macromolecules 2000, 33, 7328–7336, .
- Turrin, C.O.; Chiffre, J.; Daran, J.C.; de Montauzon, D.; Caminade, A.M.; Manoury, E.; Balavoine, G.; Majoral, J.P.; New chiral phosphorus-containing dendrimers with ferrocenes on the periphery. Tetrahedron 2001, 57, 2521–2536, .
- Routaboul, L.; Vincendeau, S.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Daran, J.C.; Manoury, E.; New phosphorus dendrimers with chiral ferrocenyl phosphine-thioether ligands on the periphery for asymmetric catalysis. J. Organomet. Chem. 2007, 692, 1064–1073, .
- Neumann, P.; Dib, H.; Sournia-Saquet, A.; Grell, T.; Handke, M.; Caminade, A.M.; Hey-Hawkins, E.; Ruthenium Complexes with Dendritic Ferrocenyl Phosphanes: Synthesis, Characterization, and Application in the Catalytic Redox Isomerization of Allylic Alcohols. Chem.-Eur. J. 2015, 21, 6590–6604, .
- Riant, O.; Samuel, O.; Flessner, T.; Taudien, S.; Kagan, H.B.; An efficient asymmetric synthesis of 2-substituted ferrocenecarboxaldehydes. J. Org. Chem. 1997, 62, 6733–6745, .
- Neumann, P.; Dib, H.; Caminade, A.M.; Hey-Hawkins, E.; Redox Control of a Dendritic Ferrocenyl-Based Homogeneous Catalyst. Angew. Chem. Int. Ed. 2015, 54, 311–314, .
- Turrin, C.O.; Chiffre, J.; Daran, J.C.; de Montauzon, D.; Balavoine, G.; Manoury, E.; Caminade, A.M.; Majoral, J.P.; New phosphorus-containing dendrimers with ferrocenyl units in each layer. C. R. Chim. 2002, 5, 309–318, .
- Turrin, C.O.; Chiffre, J.; de Montauzon, D.; Balavoine, G.; Manoury, E.; Caminade, A.M.; Majoral, J.P.; Behavior of an optically active ferrocene chiral shell located within phosphorus-containing dendrimers. Organometallics 2002, 21, 1891–1897, .
- de Jong, E.R.; Manoury, E.; Daran, J.C.; Turrin, C.O.; Chiffre, J.; Sournia-Saquet, A.; Knoll, W.; Majoral, J.P.; Caminade, A.M.; Synthesis and characterization of water-soluble ferrocene-dendrimers. J. Organomet. Chem. 2012, 718, 22–30, .
- Popp, J.; Caminade, A.M.; Hey-Hawkins, E.; Redox‑Switchable Transfer Hydrogenations with P‑Chiral Dendritic Ferrocenyl Phosphine Complexes. Eur J. Inorg. Chem. 2020, , , .
