The applications of Copper-based nanoparticles have received great attention due to the earth-abundant, low toxicity and inexpensive. Due to these characteristics, copper nanoparticles have generated a great deal of interest especially in the field of catalysis. Traditional Ullmann-type couplings suffer from limited substrate scopes and harsh reaction conditions. The introduction of a new copper-based catalyst over the past two decades has totally changed this situation as it enables the reaction promoted in mild condition. The reaction scope has also been greatly expanded, rendering this copper-based cross-coupling attractive for both academia and industry.
Transition metal-catalyzed chemical transformation of organic electrophiles and organometallic reagents belong to the most important cross-coupling reaction in organic synthesis. The biaryl ether division is not only popular in natural products and synthetic pharmaceuticals but also widely found in many pesticides, polymers, and ligands. Copper catalyst has received great attention owing to the low toxicity and low cost. The introduction of homogeneous copper catalysts with the presence of bidentate ligands and also heterogeneous copper catalyst over the past two decades has totally changed this situation as these ligands enable the reaction promoted in mild condition. The reaction scope has also been greatly expanded, rendering this copper-based cross-coupling attractive for both academia and industry. This review had been summarized recently advance homogeneous and heterogeneous copper catalyst in Ullmann reaction and its application and natural product and pharmaceutical industry.
- heterogenous catalyst
- Homogenous catalyst
- Ullmann cross coupling reaction
- Copper catalyst
- C-O bond
1. Introduction
The transition metal-catalyzed chemical transformation of organic electrophiles and organometallic reagents has turned up as an exceedingly robust synthetic tool. The evolution of transition metal catalysts has attained a stage of civilization that authorizes for an extensive scope of chemical bonds formation partners to be combined efficiently [1]. In the last century, C-C bond transformation (catenation) has permitted chemists to assemble intricate molecular frameworks of diversified interests encompassing complete synthesis of natural products, pharmaceuticals, and industrial process improvement, as well as biochemistry, materials, and nanotech [2][3]. The evolution of chemicals through the transition-metal catalyzed reaction is an exciting era for the chemistry of palladium [4], ruthenium [5], platinum [6], etc., and has been significantly adopted by the industry. For example, ruthenium-catalyzed N-H insertion reaction for the formation of benzo fused six/five-membered azaheterocycles [7] which have a wide range of significant biological activity, such as anti-bacterial, -fungal, -tubercular, helminthic, -plasmodial, -cancer, -inflammatory, cardiotonic, -hypertensive, -thrombotic, histaminic, -ulcer, analgesic, neuroleptic, etc. [8]. In contrast, the low cost and high abundance of copper metal seemed to be the left in the cold as a trend of a breakthrough in this area. However, the copper-mediated C-C, C-O, C-N, and C-S bond formation is a part of one oldest reaction, emphasizing the Ullmann cross-coupling reaction [9][10].
In the early 20th century, Ullmann and Goldberg have reported the first and primary cleavage of an aromatic carbon-halide bond in the presence of a stoichiometric amount of copper as a catalyst [11][12]. In this reaction, two moles of aryl halides are coupled in the presence of a stoichiometric amount of copper salt to afford the homocoupling product at 210–260 °C (Scheme 1) [12][13]. Even though these homocoupling reactions received considerable attention from the synthetic industry, it contributed several applications in that century. Still, this reaction suffered from harsh conditions, which included high temperature, strong base, a large amount of copper catalyst, limited substrate scope, etc. [14]. Later, Ullmann and Goldberg also reported a copper-catalyzed C-N [15] and C-O heteroatomic bonds formation reaction [16]. In 1929, Hurtley found that 3-bromobenzoic acid might be utilized for the formation of C-C bond through the homocoupling reaction [17][18] in the presence of copper-bronze and copper acetate in diketones and malonates. Unfortunately, this reaction generally needs harsh conditions, like the Ullmann and Goldberg reaction, and inadequate functional groups tolerance have restricted the applications of Ullmann and Ullmann-type reactions through their study.
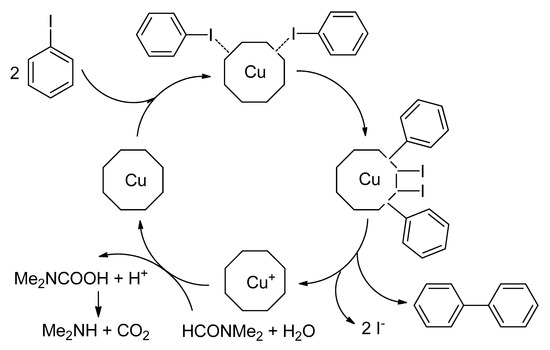
Scheme 1. Typical copper-mediated Ullmann condensation reaction and presumptive mechanism.
To modernize this prominent and classical Ullmann reaction, it is essential to establish a novel composition and morphology of catalyst, suitable solvents, and reducing agents. Back in 1970, many researchers applied various metal-based catalysts involved in Ullmann's reaction, and most included palladium, nickel, and gold [19]. However, palladium and nickel were found to be the best catalysts for Ullmann reaction due to high reactivity and regioselectivity. Unfortunately, metal catalysts have several drawbacks, such as high cost, highly toxic, and generally required poisonous organic phosphines as a stabilizing ligand [20]. Gold is the most stable metal and exhibits marvelous features, such as assemblage of different types involving materials science, the department of its own particles, size-related electronic, magnetic, and optical properties, and utilization as catalysts in biology [21]. Besides these precious metals, silver [22], nickel [23][24], and rhodium [25] were also studied and applied to the Ullmann coupling reaction. The cooperative stimulus of the polymetallic unlocked different view to the field of catalysis when a unique constituent is adequately investigated. Bimetallic catalysts revealed outstanding performance than the monometallic catalysts for their catalytic performance, stability, and selectivity [26].
The research on Ullmann's reaction is a persistent process and has adapted interesting reactions today. Under this framework and history, it is so valuable for us to investigate the reactivity and mechanism of Ullmann's reactions. These can assist us in the scheme of the new vital catalysts for the breakthrough of the related field. In the meantime, countless work has been put in to explore the Ullmann reaction process at the molecular level.
2. Aryl C-O Bond Formation Catalyzed by Copper Metal
2.1. Heterogeneous Catalyst
A heterogeneous catalyst is referred to as a catalytic reaction where the catalyst has the phase difference with the reactants. In short, it is an insoluble catalyst in a solution. A heterogeneous catalyst generally continues creating the active site with the reactant under suitable reaction conditions. These sites change the rates of chemical reactions of the reactants localized on them without changing the thermodynamic equilibrium between the materials [27].
2.2. Homogeneous Catalyst
A homogeneous catalyst is referred to as a catalytic reaction where the catalyst has the same phase with the reactant. In short, it is a soluble catalyst in a solution [28]. Homogeneous catalyst generally provides short reaction time and needs a few amounts of catalyst in the catalytic system owing to it can contact with reactant adequately compare to the heterogeneous catalyst [29].
3. Recent Application of Synthetic Ethers in Pharmaceutical and Natural Product
The aromatic C-O bonds are widely introduced in natural products and pharmaceutic-like molecules. Although the Pd-catalyzed cross-coupling reaction is a powerful system in the synthesis of this type of compounds, a constitutional problem, such as high cost and high toxicity, might restrict its application in the pharmaceutical and medicinal industry. Back to front, the current breakthrough and growing of copper-mediated Ullmann etherification reactions offer an optional and practical selection.
4. Conclusion
Ullmann C-O bond arylation reaction has become dominant and plays a vital role in organic synthesis, medicinal, agrochemical chemistry, with its being present in a broad range of natural products and biologically active compounds. Copper-mediated Ullmann reactions were virtuously developed for some time by utilizing novel ligands and synthetic ancillary tools. In this review, we summarized some recent advances and applications of the copper-mediated Ullmann coupling reaction in the synthesis of heterocycles, pharmaceutical molecules, and natural products. A marvelous agreement of work has been reliable for establishing a more effortless and fast method of Ullmann type C-O bond formation, mainly capital on catalysts. Among many novels and speedy breakthrough of this reaction, we understand the eco-synthetic methodologies, such as nanosized metal-, ligand-, additive-free condition, and the reusable heterogeneous catalyst will develop in a parallel line to significantly give impact in this Ullmann O-arylation cross-coupling reaction. We notice that the involvement of low catalyst loading (<1 mol%) in the reaction is invariably limited with aryl chlorides, and tosylates are substantially expelled as the initial substrate in most of the situation. These challenges obligate the synthetic organic transformation to study further and explore with develop a novel method and catalytic system to extend the scope and generality of the century-old Ullmann condition. As illustrated, the use of all kinds of various copper-mediated catalysts in Ullmann O-arylation allows for rapid transformations under relatively mild and ligand-free conditions, with the benefits of the excellent yield of products with the ease of catalyst separation and recover. As a summary, the outcome of most of the reactions highly depended on different diverse variables, and their connection has not been confirmed yet. The following are the tabulated part affecting the Ullmann O-arylation cross-coupling reaction.
-
Ion form of copper: Either metallic copper, Cu(I), Cu(II), or Cu(0) salts and oxides have been applied, but Cu(I) salts commonly provide the extraordinary performance.
-
Amount of copper: Commonly in the scope of 5–15 mol% based on the substrate, yet as a typical order higher loaded of copper provides a faster rate of reaction with an excellent outcome.
-
Ligand form: Bi-dentate ligands are generally chosen, and the pyridine nucleus, secondary or tertiary amines, carbonyl groups, and imino-groups are generally suitable working ligand moieties; phosphine ligands are generally not very active.
-
Ligand loaded: Bi-dentate ligands are used on average in a ratio of (copper: ligand); 1:1 or 1:2, while most of the conditions, a higher ratio leading a better outcome.
-
Base: Organic bases, such as amines, do not work well with C-O Ullmann etherification. On the other hand, inorganic bases, such as potassium phosphate or carbonate and cesium carbonate, give better results in the reaction. The most general loading of the base is 3 equivalents relative to the substrate.
-
Solvent: Depending on the reaction and reactant used, polar/non-polar solvents give a better outcome; DMF, DMSO, toluene, or acetonitrile are among the most used; N-Methyl-2-pyrrolidone (NMP) is basically utilized in microwave reactions.
-
Temperature: The comment temperature in Ullmann etherification is in the range 70–120 °C, but some cases also conduct at room temperature; a better outcome of the product is generally in higher temperatures.
-
Aryl halide: The reactivity of the aryl halide follows the trend: I > Br > Cl; the reactivity of aryl-chlorides can be activated via the strong electron-withdrawing group as substituents, ortho position of substituents/adding a source of I- the reaction (ion exchange reactions are catalyzed by Cu).
-
Nucleophile: The better the nucleophile, the better the results, such as amines/thiols> phenol; amides are more active than imides.
-
Steric hindrance: A noticeable sensitivity is usually observed, both on the aryl halide and the nucleophile. For example, the presence of the methyl group in the ortho position to the nucleophilic site can significantly reduce the corresponding product.
-
Atmosphere: Usually an inert condition; nitrogen/argon atmosphere gives a better organic transformation in Cu-catalyzed ether bond couplings.
This entry is adapted from the peer-reviewed paper 10.3390/catal10101103
References
- Ayogu, J.I.; Onoabedje, E.A. Recent advances in transition metal-catalysed cross-coupling of (hetero)aryl halides and analogues under ligand-free conditions. Catal. Sci. Technol. 2019, 9, 5233–5255, doi:10.1039/C9CY01331H.
- Ciulla, M.G.; Zimmermann, S.; Kumar, K. Cascade reaction based synthetic strategies targeting biologically intriguing indole polycycles. Org. Biomol. Chem. 2019, 17, 413–431.
- Boström, J.; Brown, D.G.; Young, R.J.; Keserü, G.M. Expanding the medicinal chemistry synthetic toolbox. Nat. Rev. Drug Discov. 2018, 17, 709–727.
- Tahmasbi, B.; Ghorbani-Choghamarani, A.; Moradi, P. Palladium fabricated on boehmite as an organic–inorganic hybrid nanocatalyst for C–C cross coupling and homoselective cycloaddition reactions. New J. Chem. 2020, 44, 3717–3727, doi:10.1039/C9NJ06129K.
- Mäkelä, E.; González Escobedo, J.L.; Lindblad, M.; Käldström, M.; Meriö-Talvio, H.; Jiang, H.; Puurunen, R.L.; Karinen, R.J.C. Hydrodeoxygenation of levulinic acid dimers on a zirconia-supported ruthenium catalyst. Catalysts 2020, 10, 200.
- Hasija, D.C.; Gopalakrishnan, J.; Mishra, A.V.; Ghase, V.D.; Patil, V.R.J.S.A.S. Exploring copper as a catalyst for cost effective synthesis of polyfluorenes: An alternative to platinum and palladium. SN Appl. Sci. 2020, 2, 1–9.
- Padín, D.; Varela, J.A.; Saá, C. Ruthenium-Catalyzed Tandem Carbene/Alkyne Metathesis/N–H Insertion: Synthesis of Benzofused Six-Membered Azaheterocycles. Org. Lett. 2020, 10.1021/acs.orglett.0c00596, doi:10.1021/acs.orglett.0c00596.
- Amariucai-Mantu, D.; Mangalagiu, V.; Danac, R.; Mangalagiu, I.I. Microwave Assisted Reactions of Azaheterocycles Formedicinal Chemistry Applications. Molecules 2020, 25, 716.
- Roundtable, N.R.C.C.S. Replacing Critical Materials with Abundant Materials. In The Role of the Chemical Sciences in Finding Alternatives to Critical Resources: A Workshop Summary; National Academies Press, US: 2012.
- Fan, Q.; Zhu, J.; Gottfried, J.M. On-Surface Ullmann Reaction for the Synthesis of Polymers and Macrocycles. In On-Surface Synthesis II; Springer, Switzerland: 2018; pp. 83–112.
- Abyazisani, M.; Jayalatharachchi, V.; MacLeod, J. 2.14—Directed On-Surface Growth of Covalently-Bonded Molecular Nanostructures. In Comprehensive Nanoscience and Nanotechnology, 2nd ed.; Andrews, D.L., Lipson, R.H., Nann, T., Eds.; Academic Press: Oxford, UK, 2019; pp. 299–326, doi:10.1016/B978-0-12-803581-8.09239-0.
- Ullmann, F.; Bielecki, J. Ueber Synthesen in der Biphenylreihe. Ber. Dtsch. Chem. Ges. 1901, 34, 2174–2185, doi:10.1002/cber.190103402141.
- Gong, X.; Wu, J.; Meng, Y.; Zhang, Y.; Ye, L.-W.; Zhu, C.J.G.C. Ligand-free palladium catalyzed Ullmann biaryl synthesis:‘household’reagents and mild reaction conditions. Green Chem. 2019, 21, 995–999.
- Jiang, J.; Du, L.; Ding, Y. Aryl-Aryl Bond Formation by Ullmann Reaction: From Mechanistic Aspects to Catalyst. Mini Rev. Org. Chem. 2020, 17, 26–46.
- Gurjar, K.K.; Sharma, R.K.J.H. Synthetic and computational studies on CuI/ligand pair promoted activation of C (Aryl)-Cl bond in C–N coupling reactions. Heliyon 2020, 6, e03233.
- Rovira, M.; Soler, M.; Guell, I.; Wang, M.-Z.; Gomez, L.; Ribas, X. Orthogonal Discrimination among Functional Groups in Ullmann-Type C–O and C–N Couplings. J. Org. Chem. 2016, 81, 7315–7325.
- Cirigottis, K.A.; Ritchie, E.; Taylor, W.C. Studies on the Hurtley reaction. Aust. J. Chem. 1974, 27, 2209–2228.
- Evano, G.; Blanchard, N. Copper-Mediated Cross-Coupling Reactions; John Wiley & Sons, New Jersey: 2013.
- Khan, F.; Dlugosch, M.; Liu, X.; Banwell, M.G. The Palladium-Catalyzed Ullmann Cross-Coupling Reaction: A Modern Variant on a Time-Honored Process. Acc. Chem. Res. 2018, 51, 1784–1795, doi:10.1021/acs.accounts.8b00169.
- Egorova, K.S.; Ananikov, V.P. Toxicity of Metal Compounds: Knowledge and Myths. Organometallics 2017, 36, 4071–4090, doi:10.1021/acs.organomet.7b00605.
- Tepale, N.; Fernández-Escamilla, V.V.A.; Carreon-Alvarez, C.; González-Coronel, V.J.; Luna-Flores, A.; Carreon-Alvarez, A.; Aguilar, J. Nanoengineering of Gold Nanoparticles: Green Synthesis, Characterization, and Applications. Crystals 2019, 9, 612.
- Prabusankar, G.; Babu, C.N.; Raju, G.; Sampath, N. Silver (I) and copper (II)-imidazolium carboxylates: Efficient catalysts in Ullmann coupling reactions. J. Chem. Sci. 2017, 129, 553–559.
- Rosen, B.M.; Quasdorf, K.W.; Wilson, D.A.; Zhang, N.; Resmerita, A.-M.; Garg, N.K.; Percec, V. Nickel-Catalyzed Cross-Couplings Involving Carbon−Oxygen Bonds. Chem. Rev. 2011, 111, 1346–1416, doi:10.1021/cr100259t.
- Tobisu, M.; Chatani, N. Cross-Couplings Using Aryl Ethers via C–O Bond Activation Enabled by Nickel Catalysts. Acc. Chem. Res. 2015, 48, 1717–1726, doi:10.1021/acs.accounts.5b00051.
- van der Boom, M.E.; Liou, S.-Y.; Ben-David, Y.; Shimon, L.J.W.; Milstein, D. Alkyl− and Aryl−Oxygen Bond Activation in Solution by Rhodium(I), Palladium(II), and Nickel(II). Transition-Metal-Based Selectivity. J. Am. Chem. Soc. 1998, 120, 6531–6541, doi:10.1021/ja9738889.
- Sun, Y.; Yang, Z.; Tian, P.; Sheng, Y.; Xu, J.; Han, Y.-F. Oxidative degradation of nitrobenzene by a Fenton-like reaction with Fe-Cu bimetallic catalysts. Appl. Catal. B Environ. 2019, 244, 1–10.
- Schlögl, R. Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2015, 54, 3465–3520, doi:10.1002/anie.201410738.
- Ke, J. Semiconductor Nanocrystals for Environmental Catalysis. In Advanced Nanomaterials for Pollutant Sensing and Environmental Catalysis. 2020, pp. 119–163.
- Shende, V.S.; Saptal, V.B.; Bhanage, B.M. Recent Advances Utilized in the Recycling of Homogeneous Catalysis. Chem. Rec. 2019, 19, 2022–2043, doi:10.1002/tcr.201800205.
