Alzheimer’s disease (AD), the most familiar type of dementia, is a severe concern in modern healthcare. Around 5.5 million people aged 65 and above have AD, and it is the sixth leading cause of mortality in the US. AD is an irreversible, degenerative brain disorder characterized by a loss of cognitive function and has no proven cure. Deep learning techniques have gained popularity in recent years, particularly in the domains of natural language processing and computer vision. Since 2014, these techniques have begun to achieve substantial consideration in AD diagnosis research, and the number of papers published in this arena is rising drastically. Deep learning techniques have been reported to be more accurate for AD diagnosis in comparison to conventional machine learning models.
- Alzheimer’s disease
- deep learning
- biomarkers
- positron emission tomography
- Magnetic Resonance Imaging
- mild cognitive impairment
1. Introduction
2. DL for AD Diagnosis
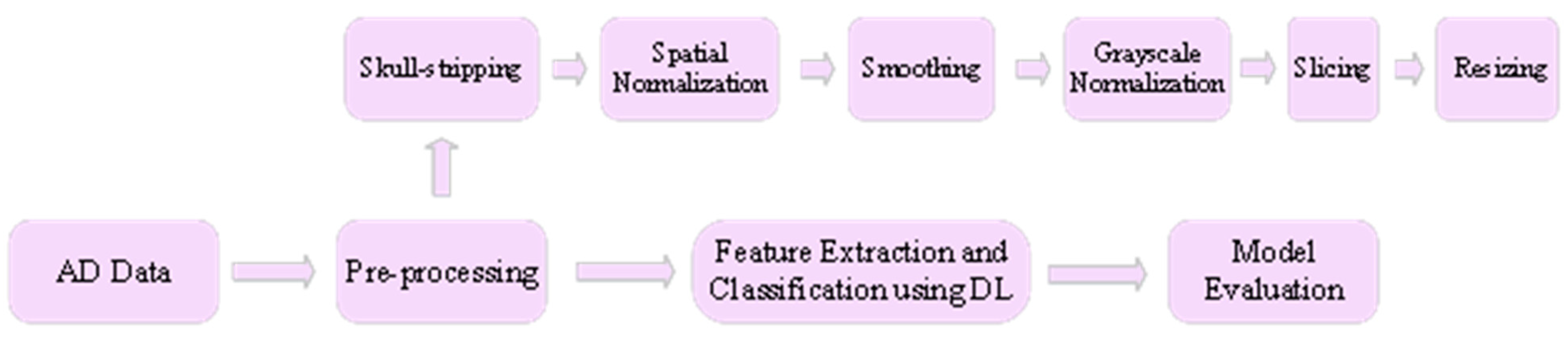
2.1. Feed-Forward DNN for AD Diagnosis
2.2. CNN for AD Diagnosis
2.3. AE for AD Diagnosis
2.4. RNN for AD Diagnosis
2.5. DBN for AD Diagnosis
2.6. GAN for AD Diagnosis
2.7. Hybrid DL Models for AD Diagnosis
| Work | Year | Biomarker | DL Method | Dataset | Performance |
|---|---|---|---|---|---|
| [45] | 2014 | MRI and PET | SAE | ADNI-311 subjects (AD-65, cMCI-67, ncMCI-102, NC-77) | Accuracy (NC/AD): 87.76% Accuracy (NC/MCI): 76.92% |
| [50] | 2015 | MRI | Residual Self Attention 3D Convolutional Neural Network | ADNI-835 subjects (AD-200, MCI-404, NC-231) | Accuracy (NC/AD): 91.3% ± 0.012 Accuracy (sMCI/pMCI): 82.1% ± 0.092 |
| [51] | 2015 | MRI | CNN + Sparse AE | ADNI-2265 subjects (AD-755, MCI-755, HC-755) | Accuracy (HC/MCI/AD): 89.47% Accuracy (HC/AD): 95.39% Accuracy (AD/MCI): 86.84% Accuracy (HC/MCI): 92.11% |
| [18] | 2016 | MRI | CNN | ADNI-805 subjects (AD-186, MCI-393, NC-226) | Accuracy (NC/ADI): 91.02% ± 4.29 Accuracy (NC/MCI): 73.02% ± 6.44 Accuracy (sMCI/pMCI): 74.82% ± 6.80 |
| [19] | 2016 | MRI | CNN | ADNI-900 subjects (AD-300, MCI-300, HC-300) | Accuracy (HC/MCI/AD): 91.85% |
| [20] | 2016 | fMRI | CNN | ADNI-43 subjects (AD-28, NC-15) | Accuracy(NC/AD): 96.85% |
| [48] | 2016 | MRI and fMRI | DBN | ADNI-275 subjects (AD-70, MCI-111, LMCI-26, NC-68) | Accuracy (NC/AD): 90% Accuracy (MCI/AD): 84% Accuracy (NC/MCI): 83% |
| [52] | 2016 | MRI | CNN + AE | ADNI-210 subjects (AD-70, MCI-70, NC-70) | Accuracy (NC/MCI/AD): 89.1% |
| [21] | 2016 | MRI | CNN | ADNI-302 subjects (AD-211, HC-91) | Accuracy (HC/AD): 98.84% |
| [22] | 2016 | MRI and fMRI | CNN | ADNI (fMRI-144 subjects: AD-52, CN-92) ADNI(MRI-302 subjects: AD-211, CN-91) |
Accuracy (fMRI (CN/AD)): 99.9% Accuracy (MRI (CN/AD)): 98.84% |
| [6] | 2017 | MRI | DNN | ADNI-240 subjects (AD-60, cMCI-60, MCI-60,HC-60) | Accuracy (HC/MCI/cMCI/AD): 53.7 ± 1.9% |
| [23] | 2017 | MRI | CNN | ADNI-504 subjects (AD-101, MCI-234, CN-169) | Accuracy (CN/MCI/AD): 96% |
| [46] | 2018 | MRI and FDG-PET | SAE | ADNI-1051 subjects (NC-304, sMCI-409, pMCI-112, AD-226) | Accuracy (NC/AD): 93.58%, Accuracy (sMCI/pMCI): 81.55% |
| [7] | 2018 | EEG | DNN | Data collected from Chosun University Hospital and Gwangju Optimal Dementia Center located in South Korea-20 subjects (MCI-10, HC-10) | Accuracy (NC/MCI): 59.3% |
| [44] | 2018 | MRI and FDG-PET images | SAE | ADNI-1242 subjects (sNC-360, sMCI-409, pNC: 18, pMCI-217, sAD-238) | Accuracy (sMCI/pMCI): 82.93% |
| [24] | 2018 | MRI | CNN | ADNI-1409 subjects (AD-294, MCI-763, HC-352), Milan dataset-229 subjects (AD-124, MCI-50, HC-55) | Accuracy (HC/AD): 98.2% Accuracy (HC/cMCI): 87.7% Accuracy (HC/sMCI): 76.4% Accuracy (cMCI/AD): 75.8% Accuracy (sMCI/AD): 86.3% Accuracy (cMCI/sMCI): 74.9% |
| [8] | 2018 | MRI and AV-45 PET data | DNN | ADNI-896 subjects (CN-248, AD-149, EMCI-296, LMCI-193) | Accuracy (CN/EMCI): 84% Accuracy (CN/LMCI): 84.1% Accuracy (CN/AD): 96.8% Accuracy (EMCI/LMCI): 69.5% Accuracy (EMCI/AD): 90.3% Accuracy (LMCI/AD): 80.2% |
| [9] | 2018 | EEG | DNN | Data collected from Medical Universities of Graz, Innsbruck and Vienna, as well as Linz General Hospital—188 subjects (Probable AD-133, Possible AD-55) | Mean Squared Error (Probable AD/Possible AD): 12.17 |
| [53] | 2018 | MRI and FDG-PET | AE + CNN | ADNI-615 subjects (AD-193, MCI-215, NC-207) | Accuracy (MCI/AD): 93% Accuracy (NC/MCI): 95% Accuracy (NC/AD): 98.8% Accuracy (NC/MCI/AD): 91.13% |
| [25] | 2018 | MRI | CNN | OASIS dataset-126 subjects (AD-28, HC-98) and data from local hospitals-70 subjects (AD-70) | Accuracy (HC/AD): 97.65% |
| [26] | 2018 | MRI | CNN | ADNI-1728 subjects (AD-346, MCI-450, LMCI-358, NC-574) | Accuracy (NC/AD): 94% Accuracy (NC/MCI): 90% Accuracy (NC/MCI/AD): 87% |
| [27] | 2018 | MRI | CNN | ADNI-391 subjects (AD-150, MCI-129, NC-112) | Accuracy (NC/AD): 96.81% Accuracy (MCI/AD): 88.43% Accuracy (NC/MCI): 92.62% Accuracy (NC/MCI/AD): 91.32% |
| [10] | 2018 | Speech transcripts | DNN | DementiaBank dataset | AUC (MCI/AD): 0.815 |
| [28] | 2018 | MRI, clinical assessment and genetic (APOe4) measures | CNN | ADNI-800 subjects (AD-200, MCI-400, NC-200) | Accuracy (NC/MCI/AD): 99% |
| [29] | 2018 | fMRI and Diffusion Tensor Imaging (DTI) | CNN | ADNI-105 subjects (AD-35, aMCI-30, NC-40) | Accuracy (NC/aMCI/AD): 92.06% |
| [54] | 2018 | Speech transcripts | Gated CNN | DementiaBank dataset-267 subjects (AD-169, HC-98) | Accuracy (HC/AD): 73.6% |
| [11] | 2018 | MRI and single nucleotide polymorphism (SNP) data | DNN | ADNI-721 subjects (AD-138, MCI-358, CN-225) | AUC (CN/MCI/AD): 0.992 |
| [30] | 2018 | MRI | CNN | OASIS dataset-416 subjects | Accuracy (Non Demented/very Mild/Mild/Moderate): 93% |
| [55] | 2018 | MRI and PET | CNN + RNN | ADNI-397 subjects (AD-93, pMCI-76, sMCI-128, CN-100) | Accuracy (NC/AD): 94.29% Accuracy (NC/pMCI): 84.66% Accuracy (NC/sMCI): 64.47% |
| [31] | 2018 | MRI | CNN | ADNI-1663 subjects (AD-336, MCI-542, CN-785) | Accuracy (NC/LMCI): 94.5% Accuracy (NC/AD): 96.9% Accuracy (LMCI/AD): 97.2% Accuracy (EMCI/AD): 97.81% Accuracy (EMCI/LMCI): 94.8% |
| [12] | 2019 | gene expression and DNA methylation profiles | DNN | GSE33000 and GSE44770 (gene expression), prefrontal cortex GSE80970 (DNA methylation) | Accuracy (NC/AD): 82.3% |
| [13] | 2019 | MRI | DNN | OASIS-416 subjects | Accuracy (NC/AD): 86.66% |
| [32] | 2019 | MRI | CNN | ADNI-150 subjects (AD-50, CN-50, MCI-50) | Accuracy (CN/AD): 99.14% Accuracy (AD/MCI): 99.3% Accuracy (CN/MCI): 99.2% |
| [14] | 2019 | MRI | DNN | ADNI-291 subjects (AD-97, CN-194) | Accuracy (CN/AD): 67% |
| [56] | 2019 | MRI | CNN + RNN | ADNI-807 subjects (AD-194, MCI-397, NC-216) | Accuracy (NC/AD): 91.0% Accuracy (NC/MCI): 75.8% Accuracy (sMCI/pMCI): 74.6% |
| [57] | 2019 | MRI | AE+ CNN | ADNI-694 subjects (AD-198, NC-230, sMCI-101, pMCI-166) | Accuracy (AD/NC): 86.60% ± 3.66% Accuracy (pMCI/NC): 77.37% ± 3.55% Accuracy (sMCI/NC): 63.04% ± 4.16% Accuracy (pMCI/AD): 60.97% ± 5.33% Accuracy (sMCI/AD): 75.06% ± 3.86 |
| [33] | 2019 | MRI and FDG-PET | CNN | ADNI-2145 subjects (AD-647, sMCI-441, pMCI-326, HC-731) | Accuracy (NC/AD): 90.10% Accuracy (NC/pMCI): 87.46% Accuracy (sMCI/pMCI): 76.90% |
| [34] | 2019 | MRI | CNN | ADNI-315 subjects (AD-185, HC-130) | Accuracy (HC/AD): 98.06% |
| [47] | 2019 | Demographic information, neuro-imaging phenotypes measured by MRI, cognitive performance, and CSF measurements | RNN | ADNI-1618 subjects (AD-338, MCI-865, CN-415) | Accuracy (CN/MCI/AD): 81% |
| [58] | 2019 | Speech transcripts | CNN + RNN | DementiaBank dataset | AUC (NC/AD): 0.838 |
| [59] | 2019 | MRI | CNN + AE | ADNI-1941 subjects (AD-345, MCI-991, NC-605) | Accuracy (MCI/AD): 94.6% Accuracy (NC/AD): 92.98% Accuracy (NC/MCI): 94.04% |
| [35] | 2019 | MRI and PET | CNN | ADNI-392 subjects (AD-91, MCI-200, CN-101) | Accuracy (NC/AD): 98.47% Accuracy (NC/MCI): 85.74% Accuracy (AD/MCI): 88.20% |
| [36] | 2019 | MRI | CNN | ADNI-1820 images (AD-635, MCI: 548, CN: 637) | Accuracy (CN/MCI/AD): 86.9% Accuracy (CN/AD): 100% Accuracy (MCI/AD): 96.2% Accuracy (CN/MCI): 98% |
| [15] | 2019 | MRI | DNN | ADNI-1737 subjects | AUC (NC/MCI/AD): 0.866 |
| [37] | 2019 | MRI and clinical features | CNN | ADNI-785 subjects (AD-192, MCI-409, HC-184) | Accuracy (MCI/AD): 86% |
| [49] | 2020 | MRI | GAN | ADNI-1114 subjects and Frontotemporal Lobar Degeneration Neuroimaging Initiative (NIFD)-840 subjects | Accuracy (NC/AD): 88.28% |
| [38] | 2020 | MRI | CNN | ADNI | Test time (NC/AD): 0.2 s |
| [39] | 2020 | MEG | CNN | Data collected from Centre for Biomedical Technology, Spain-132 subjects (MCI-78, HC-54) | F1-Score (HC/MCI) = 0.92 |
| [40] | 2020 | MRI | CNN | OASIS dataset-126 subjects (AD-28, HC-98) and data from local hospitals-70 subjects (AD-70) | Accuracy (HC/AD): 97.76% ± 0.41 |
| [41] | 2020 | MRI | CNN | ADNI-159 subjects (AD-45, MCI-62, NC-52) | Accuracy (NC/MCI/AD): 99.89% |
| [42] | 2020 | MRI | CNN | ADNI-390 subjects (AD-195, CN-195), SNUBH-390 subjects (AD-195, CN-195) | Accuracy (ADNI (CN/AD)): 89% Accuracy (SNUBH (CN/AD)): 88% |
| [63] | 2020 | fMRI and PET | CNN | fMRI ADNI dataset-54 subjects (AD-27, HC-27) PET ADNI dataset-2675 images (AD-900, HC-1775) |
Accuracy (fMRI dataset (HC/AD)): 99.95% Accuracy (PET ADNI (HC/AD)): 73.46% |
| [64] | 2020 | MRI | CNN | Kaggle’s MRI dataset | Accuracy (MCI/AD): 96% |
| [16] | 2020 | MRI | DNN | ADNI-819 subjects (AD-192, MCI-398, CN-229) and NIMHANS-99 (AD-39, CN-60) | Accuracy (ADNI (CN/MCI/AD)): 99.50% Accuracy (NIMHANS (CN/AD)): 98.40% |
| [65] | 2020 | MRI | CNN | OASIS-382 images (No Dementia: 167, Very Mild Dementia-87, Mild Dementia-105, Moderate AD-23) | Accuracy (No Dementia/Very Mild Dementia/Mild Dementia/Moderate AD): 99.05% |
| [66] | 2020 | MRI | CNN | ADNI-465 subjects (AD-132, MCI-181, CN-152) | Accuracy (CN/MCI/AD): 97.77% |
| [67] | 2020 | MRI | CNN | ADNI-132 subjects (AD-25, MCI-61, CN-46) | Accuracy (CN/MCI/AD):84% |
| [68] | 2020 | MRI | CNN | ADNI-GO/2-663 subjects ADNI-3-575 subjects AIBL-606 subjects DELCODE-474 subjects |
Accuracy (ADNI-GO/2): 86.25% Accuracy (ADNI-3): 74.375% Accuracy (AIBL): 79.225% Accuracy (DELCODE): 78% |
| [69] | 2020 | MRI | CNN | ADNI-469 subjects (AD-153, MCI-157, CN-159) | Accuracy (NC/MCI/AD): 92.11% ± 2.31 Accuracy (NC/AD): 99.10% ± 1.13 Accuracy (NC/MCI): 98.90% ± 2.78 Accuracy (MCI/AD): 89.40% ± 6.90 |
| [43] | 2020 | Tau-PET | CNN | ADNI-300 subjects (AD-66. EMCI-97, LMCI-71, CN-66) | Accuracy (CN/AD): 90.8% |
| [60] | 2021 | MRI | CNN + DNN | Gwangju Alzheimer’s and Related Dementia (GARD) | Accuracy (NC/AD): 94.02% |
| [61] | 2021 | Speech transcripts | Bidirectional encoder with logistic regression | DementiaBank dataset-269 subjects (AD-170, HC-99) | Accuracy (HC/AD): 88.08% |
| [62] | 2021 | MRI | CNN with attention mechanism | ADNI-968 subjects (AD-280, cMCI-162, ncMCI-251, NC-275) | Accuracy (NC/AD): 97.35% Accuracy (NC/MCI): 87.82% Accuracy (MCI/AD): 78.79% |
| [70] | 2021 | MRI and PET | CNN | ADNI-5556 images (AD-718, EMCI-1222, MCI-1274, LMCI-636, SMC-186, CN-1520) | Accuracy (CN/EMCI/MCI/LMCI/AD): 86% |
| [71] | 2021 | fMRI | CNN | ADNI-675 subjects | Accuracy (Low AD): 98.1% Accuracy (Mild AD): 95.2% Accuracy (Moderate AD): 89% Accuracy (Severe AD): 87.5% |
| [72] | 2021 | MRI | CNN | ADNI-450 subjects (AD-150, MCI-150, NC-150) | Accuracy (NC/AD): 90.15% ± 1.1 Accuracy (MCI/AD): 87.30% ± 1.4 Accuracy (NC/MCI): 83.90% ± 2.5 |
| [17] | 2021 | Genetic Measures | DNN | MCSA-266 subjects | p-value ˂ 1 × 10−3 |
| [73] | 2021 | FDG-PET | CNN | MCSA | Mean Absolute Error: 2.8942 |
| [74] | 2021 | MRI | CNN | HABS | Error rate < 1% |
| [75] | 2021 | Tau-PET and MRI | CNN | Tau-PET and MRI images from two human brains | Area under Curve: 0.88 |
This entry is adapted from the peer-reviewed paper 10.3390/jpm12050815
References
- Rabeh, A.B.; Benzarti, F.; Amiri, H. Diagnosis of alzheimer diseases in early step using SVM (Support Vector Machine). In Proceedings of the 2016 13th International Conference on Computer Graphics, Imaging and Visualization (CGiV), Beni Mellal, Morocco, 29 March–1 April 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 364–367.
- Alam, S.; Kwon, G.R.; Kim, J.I.; Park, C.S. Twin SVM-based classification of Alzheimer’s disease using complex dual-tree wavelet principal coefficients and LDA. J. Healthc. Eng. 2017, 2017, 1–12.
- Acharya, U.R.; Fernandes, S.L.; WeiKoh, J.E.; Ciaccio, E.J.; Fabell, M.K.M.; Tanik, U.J.; Rajinikanth, V.; Yeong, C.H. Automated detection of Alzheimer’s disease using brain MRI images–a study with various feature extraction techniques. J. Med. Syst. 2019, 43, 302.
- Tufail, A.B.; Abidi, A.; Siddiqui, A.M.; Younis, M.S. Automatic classification of initial categories of Alzheimer’s disease from structural MRI phase images: A comparison of PSVM, KNN and ANN methods. Int. J. Biomed. Biol. Eng. 2012, 6, 713–717.
- Ramírez, J.; Górriz, J.M.; Segovia, F.; Chaves, R.; Salas-Gonzalez, D.; López, M.; Álvarez, I.; Padilla, P. Computer aided diagnosis system for the Alzheimer’s disease based on partial least squares and random forest SPECT image classification. Neurosci. Lett. 2010, 472, 99–103.
- Amoroso, N.; Diacono, D.; Fanizzi, A.; La Rocca, M.; Monaco, A.; Lombardi, A.; Guaragnella, C.; Bellotti, R.; Tangaro, S.; Alzheimer’s Disease Neuroimaging Initiative. Deep learning reveals Alzheimer’s disease onset in MCI subjects: Results from an international challenge. J. Neurosci. Methods 2018, 302, 3–9.
- Kim, D.; Kim, K. Detection of early stage Alzheimer’s disease using EEG relative power with deep neural network. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 352–355.
- Forouzannezhad, P.; Abbaspour, A.; Li, C.; Cabrerizo, M.; Adjouadi, M. A deep neural network approach for early diagnosis of mild cognitive impairment using multiple features. In Proceedings of the 2018 17th IEEE International Conference on Machine Learning and Applications (ICMLA), Orlando, FL, USA, 17–20 December 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1341–1346.
- Fruehwirt, W.; Cobb, A.D.; Mairhofer, M.; Weydemann, L.; Garn, H.; Schmidt, R.; Benke, T.; Dal-Bianco, P.; Ransmayr, G.; Waser, M.; et al. Bayesian deep neural networks for low-cost neurophysiological markers of Alzheimer’s disease severity. arXiv 2018, arXiv:1812.04994.
- Orimaye, S.O.; Wong, J.S.M.; Wong, C.P. Deep language space neural network for classifying mild cognitive impairment and Alzheimer-type dementia. PLoS ONE 2018, 13, e0205636.
- Ning, K.; Chen, B.; Sun, F.; Hobel, Z.; Zhao, L.; Matloff, W.; Toga, A.W.; Alzheimer’s Disease Neuroimaging Initiative. Classifying Alzheimer’s disease with brain imaging and genetic data using a neural network framework. Neurobiol. Aging 2018, 68, 151–158.
- Park, C.; Ha, J.; Park, S. Prediction of Alzheimer’s disease based on deep neural network by integrating gene expression and DNA methylation dataset. Expert Syst. Appl. 2020, 140, 112873.
- Benyoussef, E.M.; Elbyed, A.; El Hadiri, H. 3D MRI classification using KNN and deep neural network for Alzheimer’s disease diagnosis. In International Conference on Advanced Intelligent Systems for Sustainable Development; Tangier, Morocco, 12–14 July, Springer: Cham, Switzerland, 2018; pp. 154–158.
- Manzak, D.; Çetinel, G.; Manzak, A. Automated Classification of Alzheimer’s Disease using Deep Neural Network (DNN) by Random Forest Feature Elimination. In Proceedings of the 2019 14th International Conference on Computer Science & Education (ICCSE), Toronto, ON, Canada, 19–21 August 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1050–1053.
- Albright, J.; Alzheimer’s Disease Neuroimaging Initiative. Forecasting the progression of Alzheimer’s disease using neural networks and a novel preprocessing algorithm. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 483–491.
- Suresha, H.S.; Parthasarathy, S.S. Alzheimer Disease Detection Based on Deep Neural Network with Rectified Adam Optimization Technique using MRI Analysis. In Proceedings of the 2020 Third International Conference on Advances in Electronics, Computers and Communications (ICAECC), Bengaluru, India, 11–12 December 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–6.
- Wang, Q.; Readhead, B.; Chen, K.; Su, Y.; Reiman, E.M.; Dudley, J.T. Deep Learning-Based Brain Transcriptomic Signatures Associated with the Neuropathological and Clinical Severity of Alzheimer’s Disease. Brain Commun. 2021, 4, fcab293.
- Suk, H.I.; Shen, D. Deep ensemble sparse regression network for Alzheimer’s disease diagnosis. In International Workshop on Machine Learning in Medical Imaging; Springer: Cham, Switzerland, 2016; pp. 113–121.
- Billones, C.D.; Demetria, O.J.L.D.; Hostallero, D.E.D.; Naval, P.C. DemNet: A convolutional neural network for the detection of Alzheimer’s disease and mild cognitive impairment. In Proceedings of the 2016 IEEE Region 10 Conference (TENCON), Singapore, 22–25 November 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 3724–3727.
- Sarraf, S.; Tofighi, G. Classification of alzheimer’s disease using fmri data and deep learning convolutional neural networks. arXiv 2016, arXiv:1603.08631.
- Sarraf, S.; Tofighi, G. Classification of Alzheimer’s disease structural MRI data by deep learning convolutional neural networks. arXiv 2016, arXiv:1607.06583.
- Sarraf, S.; Tofighi, G.; Alzheimer’s Disease Neuroimaging Initiative. DeepAD: Alzheimer’s disease classification via deep convolutional neural networks using MRI and fMRI. BioRxiv 2016, 070441.
- Gunawardena, K.A.N.N.P.; Rajapakse, R.N.; Kodikara, N.D. Applying convolutional neural networks for pre-detection of alzheimer’s disease from structural MRI data. In Proceedings of the 2017 24th International Conference on Mechatronics and Machine Vision in Practice (M2VIP), Auckland, New Zealand, 21–23 November 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–7.
- Basaia, S.; Agosta, F.; Wagner, L.; Canu, E.; Magnani, G.; Santangelo, R.; Filippi, M.; Alzheimer’s Disease Neuroimaging Initiative. Automated classification of Alzheimer’s disease and mild cognitive impairment using a single MRI and deep neural networks. NeuroImage Clin. 2019, 21, 101645.
- Wang, S.H.; Phillips, P.; Sui, Y.; Liu, B.; Yang, M.; Cheng, H. Classification of Alzheimer’s disease based on eight-layer convolutional neural network with leaky rectified linear unit and max pooling. J. Med. Syst. 2018, 42, 1–11.
- Karasawa, H.; Liu, C.L.; Ohwada, H. Deep 3d convolutional neural network architectures for alzheimer’s disease diagnosis. In Asian Conference on Intelligent Information and Database Systems; Springer: Cham, Switzerland, 2018; pp. 287–296.
- Tang, H.; Yao, E.; Tan, G.; Guo, X. A fast and accurate 3D fine-tuning convolutional neural network for Alzheimer’s disease diagnosis. In International CCF Conference on Artificial Intelligence; Springer: Singapore, 2018; pp. 115–126.
- Spasov, S.E.; Passamonti, L.; Duggento, A.; Liò, P.; Toschi, N. A multi-modal convolutional neural network framework for the prediction of Alzheimer’s disease. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1271–1274.
- Wang, Y.; Yang, Y.; Guo, X.; Ye, C.; Gao, N.; Fang, Y.; Ma, H.T. A novel multimodal MRI analysis for Alzheimer’s disease based on convolutional neural network. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 754–757.
- Islam, J.; Zhang, Y. Brain MRI analysis for Alzheimer’s disease diagnosis using an ensemble system of deep convolutional neural networks. Brain Inform. 2018, 5, 2.
- Yue, L.; Gong, X.; Chen, K.; Mao, M.; Li, J.; Nandi, A.K.; Li, M. Auto-detection of Alzheimer’s disease using deep convolutional neural networks. In Proceedings of the 2018 14th International Conference on Natural Computation, Fuzzy Systems and Knowledge Discovery (ICNC-FSKD), Huangshan, China, 28–30 July 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 228–234.
- Jain, R.; Jain, N.; Aggarwal, A.; Hemanth, D.J. Convolutional neural network based Alzheimer’s disease classification from magnetic resonance brain images. Cogn. Syst. Res. 2019, 57, 147–159.
- Huang, Y.; Xu, J.; Zhou, Y.; Tong, T.; Zhuang, X. Diagnosis of Alzheimer’s disease via multi-modality 3D convolutional neural network. Front. Neurosci. 2019, 13, 509.
- Goceri, E. Diagnosis of Alzheimer’s disease with Sobolev gradient-based optimization and 3D convolutional neural network. Int. J. Numer. Methods Biomed. Eng. 2019, 35, e3225.
- Zhang, F.; Li, Z.; Zhang, B.; Du, H.; Wang, B.; Zhang, X. Multi-modal deep learning model for auxiliary diagnosis of Alzheimer’s disease. Neurocomputing 2019, 361, 185–195.
- Basheera, S.; Ram, M.S.S. Convolution neural network–based Alzheimer’s disease classification using hybrid enhanced independent component analysis based segmented gray matter of T2 weighted magnetic resonance imaging with clinical valuation. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 974–986.
- Spasov, S.; Passamonti, L.; Duggento, A.; Lio, P.; Toschi, N.; Alzheimer’s Disease Neuroimaging Initiative. A parameter-efficient deep learning approach to predict conversion from mild cognitive impairment to Alzheimer’s disease. Neuroimage 2019, 189, 276–287.
- Ahmad, I.; Pothuganti, K. Analysis of different convolution neural network models to diagnose Alzheimer’s disease. Mater. Today Proc. 2020, in press.
- Lopez-Martin, M.; Nevado, A.; Carro, B. Detection of early stages of Alzheimer’s disease based on MEG activity with a randomized convolutional neural network. Artif. Intell. Med. 2020, 107, 101924.
- Jiang, X.; Chang, L.; Zhang, Y.D. Classification of Alzheimer’s disease via eight-layer convolutional neural network with batch normalization and dropout techniques. J. Med. Imaging Health Inform. 2020, 10, 1040–1048.
- Nawaz, A.; Anwar, S.M.; Liaqat, R.; Iqbal, J.; Bagci, U.; Majid, M. Deep Convolutional Neural Network based Classification of Alzheimer’s Disease using MRI Data. In Proceedings of the 2020 IEEE 23rd International Multitopic Conference (INMIC), Bahawalpur, Pakistan, 5–7 November 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–6.
- Bae, J.B.; Lee, S.; Jung, W.; Park, S.; Kim, W.; Oh, H.; Han, J.W.; Kim, G.E.; Kim, J.S.; Kim, J.H.; et al. Identification of Alzheimer’s disease using a convolutional neural network model based on T1-weighted magnetic resonance imaging. Sci. Rep. 2020, 10, 22252.
- Jo, T.; Nho, K.; Risacher, S.L.; Saykin, A.J. Deep learning detection of informative features in tau PET for Alzheimer’s disease classification. BMC Bioinform. 2020, 21, 496.
- Lu, D.; Popuri, K.; Ding, G.W.; Balachandar, R.; Beg, M.F. Multimodal and multiscale deep neural networks for the early diagnosis of Alzheimer’s disease using structural MR and FDG-PET images. Sci. Rep. 2018, 8, 5697.
- Liu, S.; Liu, S.; Cai, W.; Pujol, S.; Kikinis, R.; Feng, D. Early diagnosis of Alzheimer’s disease with deep learning. In Proceedings of the 2014 IEEE 11th International Symposium on Biomedical Imaging (ISBI), Beijing, China, 29 April–2 May 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 1015–1018.
- Lu, D.; Popuri, K.; Ding, G.W.; Balachandar, R.; Beg, M.F.; Alzheimer’s Disease Neuroimaging Initiative. Multiscale deep neural network based analysis of FDG-PET images for the early diagnosis of Alzheimer’s disease. Med. Image Anal. 2018, 46, 26–34.
- Lee, G.; Nho, K.; Kang, B.; Sohn, K.A.; Kim, D. Predicting Alzheimer’s disease progression using multi-modal deep learning approach. Sci. Rep. 2019, 9, 1952.
- Ortiz, A.; Munilla, J.; Gorriz, J.M.; Ramirez, J. Ensembles of deep learning architectures for the early diagnosis of the Alzheimer’s disease. Int. J. Neural Syst. 2016, 26, 1650025.
- Ma, D.; Lu, D.; Popuri, K.; Wang, L.; Beg, M.F.; Alzheimer’s Disease Neuroimaging Initiative. Differential Diagnosis of Frontotemporal Dementia, Alzheimer’s Disease, and Normal Aging Using a Multi-Scale Multi-Type Feature Generative Adversarial Deep Neural Network on Structural Magnetic Resonance Images. Front. Neurosci. 2020, 14, 853.
- Zhang, X.; Han, L.; Zhu, W.; Sun, L.; Zhang, D. An Explainable 3D Residual Self-Attention Deep Neural Network For Joint Atrophy Localization and Alzheimer’s Disease Diagnosis using Structural MRI. IEEE J. Biomed. Health Inform. 2021.
- Payan, A.; Montana, G. Predicting Alzheimer’s disease: A neuroimaging study with 3D convolutional neural networks. arXiv 2015, arXiv:1502.02506.
- Hosseini-Asl, E.; Keynton, R.; El-Baz, A. Alzheimer’s disease diagnostics by adaptation of 3D convolutional network. In Proceedings of the 2016 IEEE International Conference on Image Processing (ICIP), Phoenix, AZ, USA, 25–28 September 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 126–130.
- Vu, T.D.; Ho, N.H.; Yang, H.J.; Kim, J.; Song, H.C. Non-white matter tissue extraction and deep convolutional neural network for Alzheimer’s disease detection. Soft Comput. 2018, 22, 6825–6833.
- Warnita, T.; Inoue, N.; Shinoda, K. Detecting Alzheimer’s disease using gated convolutional neural network from audio data. arXiv 2018, arXiv:1803.11344.
- Feng, C.; Elazab, A.; Yang, P.; Wang, T.; Lei, B.; Xiao, X. 3D convolutional neural network and stacked bidirectional recurrent neural network for Alzheimer’s disease diagnosis. In International Workshop on Predictive Intelligence in Medicine; Springer: Cham, Switzerland, 2018; pp. 138–146.
- Li, F.; Liu, M.; Alzheimer’s Disease Neuroimaging Initiative. A hybrid convolutional and recurrent neural network for hippocampus analysis in Alzheimer’s disease. J. Neurosci. Methods 2019, 323, 108–118.
- Oh, K.; Chung, Y.C.; Kim, K.W.; Kim, W.S.; Oh, I.S. Classification and visualization of Alzheimer’s disease using volumetric convolutional neural network and transfer learning. Sci. Rep. 2019, 9, 18150.
- Chien, Y.W.; Hong, S.Y.; Cheah, W.T.; Yao, L.H.; Chang, Y.L.; Fu, L.C. An automatic assessment system for Alzheimer’s disease based on speech using feature sequence generator and recurrent neural network. Sci. Rep. 2019, 9, 19597.
- Kruthika, K.R.; Maheshappa, H.D.; Alzheimer’s Disease Neuroimaging Initiative. CBIR system using Capsule Networks and 3D CNN for Alzheimer’s disease diagnosis. Inform. Med. Unlocked 2019, 14, 59–68.
- Basher, A.; Kim, B.C.; Lee, K.H.; Jung, H.Y. Volumetric Feature-Based Alzheimer’s Disease Diagnosis From sMRI Data Using a Convolutional Neural Network and a Deep Neural Network. IEEE Access 2021, 9, 29870–29882.
- Roshanzamir, A.; Aghajan, H.; Baghshah, M.S. Transformer-based deep neural network language models for Alzheimer’s disease risk assessment from targeted speech. BMC Med. Inform. Decis. Mak. 2021, 21, 92.
- Zhang, J.; Zheng, B.; Gao, A.; Feng, X.; Liang, D.; Long, X. A 3D densely connected convolution neural network with connection-wise attention mechanism for Alzheimer’s disease classification. Magn. Reson. Imaging 2021, 78, 119–126.
- Janghel, R.R.; Rathore, Y.K. Deep Convolution Neural Network Based System for Early Diagnosis of Alzheimer’s Disease. IRBM 2021, 42, 258–267.
- Sathiyamoorthi, V.; Ilavarasi, A.K.; Murugeswari, K.; Ahmed, S.T.; Devi, B.A.; Kalipindi, M. A deep convolutional neural network based computer aided diagnosis system for the prediction of Alzheimer’s disease in MRI images. Measurement 2021, 171, 108838.
- Mehmood, A.; Maqsood, M.; Bashir, M.; Shuyuan, Y. A deep siamese convolution neural network for multi-class classification of alzheimer disease. Brain Sci. 2020, 10, 84.
- Raju, M.; Gopi, V.P.; Anitha, V.S.; Wahid, K.A. Multi-class diagnosis of Alzheimer’s disease using cascaded three dimensional-convolutional neural network. Phys. Eng. Sci. Med. 2020, 43, 1219–1228.
- Sun, J.; Yan, S.; Song, C.; Han, B. Dual-functional neural network for bilateral hippocampi segmentation and diagnosis of Alzheimer’s disease. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 445–455.
- Dyrba, M.; Hanzig, M.; Altenstein, S.; Bader, S.; Ballarini, T.; Brosseron, F.; Buerger, K.; Cantré, D.; Dechent, P.; Dobisch, L.; et al. Improving 3D convolutional neural network comprehensibility via interactive visualization of relevance maps: Evaluation in Alzheimer’s disease. arXiv 2020, arXiv:2012.10294.
- Feng, W.; Halm-Lutterodt, N.V.; Tang, H.; Mecum, A.; Mesregah, M.K.; Ma, Y.; Li, H.; Zhang, F.; Wu, Z.; Yao, E.; et al. Automated MRI-based deep learning model for detection of Alzheimer’s disease process. Int. J. Neural Syst. 2020, 30, 2050032.
- Solano-Rojas, B.; Villalón-Fonseca, R. A Low-Cost Three-Dimensional DenseNet Neural Network for Alzheimer’s Disease Early Discovery. Sensors 2021, 21, 1302.
- Amini, M.; Pedram, M.; Moradi, A.; Ouchani, M. Diagnosis of Alzheimer’s Disease Severity with fMRI Images Using Robust Multitask Feature Extraction Method and Convolutional Neural Network (CNN). Comput. Math. Methods Med. 2021, 2021, 5514839.
- Turkson, R.E.; Qu, H.; Mawuli, C.B.; Eghan, M.J. Classification of Alzheimer’s Disease Using Deep Convolutional Spiking Neural Network. Neural Processing Lett. 2021, 53, 2649–2663.
- Lee, J.; Burkett, B.; Min, H.K.; Senjem, M.; Lundt, E.; Botha, H.; Graff-Radford, J.; Barnard, L.; Gunter, J.; Schwarz, C.; et al. Deep learning-based brain age prediction in normal aging and dementia. Nat. Aging 2021, 2, 412–424.
- Greve, D.N.; Billot, B.; Cordero, D.; Hoopes, A.; Hoffmann, M.; Dalca, A.V.; Fischl, B.; Iglesias, J.E.; Augustinack, J.C. A deep learning toolbox for automatic segmentation of subcortical limbic structures from MRI images. Neuroimage 2021, 244, 118610.
- Ushizima, D.; Chen, Y.; Alegro, M.; Ovando, D.; Eser, R.; Lee, W.; Poon, K.; Shankar, A.; Kantamneni, N.; Satrawada, S.; et al. Deep Learning for Alzheimer’s Disease: Mapping Large-scale Histological Tau Protein for Neuroimaging Biomarker Validation. NeuroImage 2021, 248, 118790.
