Arsenic (As) is a naturally found crystalline metalloid with ubiquitous distribution throughout the earth’s crust. As exposure to the human food chain ecosystem comprises air, water, food, and soil. Daily diet contamination depends on the inorganic or organic forms, oxidation state, water solubility, and food matrix. The differentiation and categorization of different foods as sources of inorganic and organic As contamination in daily life is an important issue.
- arsenic
- health hazards
- water
1. Introduction
|
Different Species of Arsenic |
Abbreviation |
Distribution |
References |
|---|---|---|---|
|
Arsenocholine |
AC |
Arsenic species generally found in seafood and oxidized to arsenobetaine in a biological system. |
[6] |
|
In organic arsenic |
iAs |
Found in most foods and its presence in water is in low amounts. |
[7] |
|
Arsenite |
As (III) |
It is highly toxic in nature but present in lesser amounts in most foods. |
[7] |
|
Arsenate |
As (V) |
It is highly toxic in nature but present in lesser amounts in most foods and water. |
|
|
Dimethylarsinate |
DMA |
Found in seafood and terrestrial foods and is a urine metabolite of iAs arsenosugars. |
[10] |
|
Dimethylarsinite |
DMA (III) |
It cannot be detected in food samples. It is a metabolite of iAs and can be seen in human urine samples but is highly toxic in nature. |
[11] |
|
Methylarsonate |
MA |
Found in seafood and terrestrial foods in very low amounts and is a metabolite of iAs that can be seen in urine. |
[12] |
|
Methylarsonite |
MA (III) |
It cannot be detected in food samples. It is a metabolite of iAs that can be seen in human urine samples but it is a toxic metabolite. |
[13] |
|
Arsenobetaine Arsenosugar |
AB |
It is a major arsenic species and commonly found in seafood but is non-toxic in nature. |
[7] |
|
Trimethylarsonio propionate |
TMAP |
Present in most foods. It is one of the major arsenic species. |
[14] |
|
Trimethylarsine oxide |
TMAO |
It is generally found in seafood and distributed in small amounts. |
[11] |
2. Arsenic Cellular Metabolism
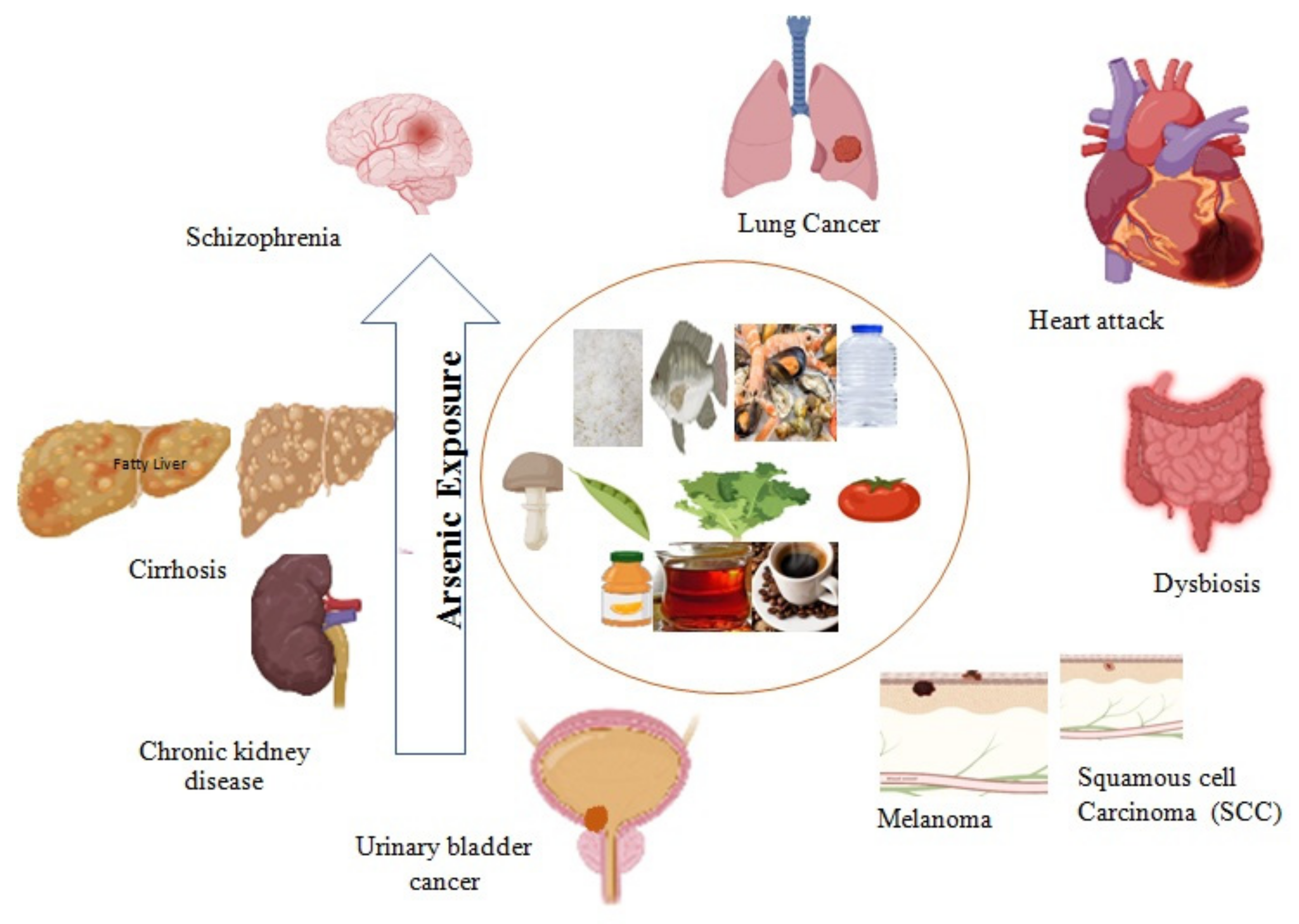
3. Arsenic-induced Health Hazards
3.1. Major Organ Damage and Chronic Disease Development
|
Arsenic Chemical Forms |
Health Effects |
References |
|---|---|---|
|
Inorganic arsenic (AsIII and AsV) |
Cancer |
[37] |
|
Chronic diseases |
||
|
Mutation |
[40] |
|
|
DNA damage |
[41] |
|
|
Mitochondrial dysfunction |
[42] |
|
|
Reduces bone mineralization |
[43] |
|
|
Hyperglycemia |
[44] |
|
|
Lipid disorders |
[45] |
|
|
Coronary heart disease |
[46] |
|
|
Liver toxicity |
[47] |
|
|
Hypertension |
[48] |
|
|
Genotoxicity |
[49] |
|
|
Arsenite (AsIII) |
Cancer |
[50] |
|
Fatty liver |
[51] |
|
|
Hepatotoxicity |
[52] |
|
|
Arsenic trioxide |
Breast cancer |
[53] |
|
Arsenic Species |
Direct Toxic Effect and Target Organ Damage (TOD) |
Molecular Mechanisms |
References |
|---|---|---|---|
|
Inorganic arsenic in drinking water and rice |
Skin cancer |
Differentiation and generation of cancer stem cells |
|
|
Coronary artery disease and cardiac muscle damage |
Cardiac tissue hypoxia and inflammation |
[61] |
|
|
Diabetes and insulin resistance |
Inhibition of glycolysis, Krebs’s cycle, and ATP synthesis |
||
|
Acute Kidney Injury (AKI) |
Kidney injury molecule-1 (KIM1) |
[64] |
|
|
Chronic kidney disease (CKD) |
Decreased glomerular filtration rate |
[65] |
|
|
Arsenite (AsIII) |
Insulin resistance and metabolic syndrome |
Diminished translocation of GLUT4 |
[66] |
|
MMA (Monomethylarsonic acid) |
Breast cancer |
Endocrine disruptor |
[67] |
|
Lung cancer |
DNA damage |
||
|
Kidney cancer |
DNA damage |
[68] |
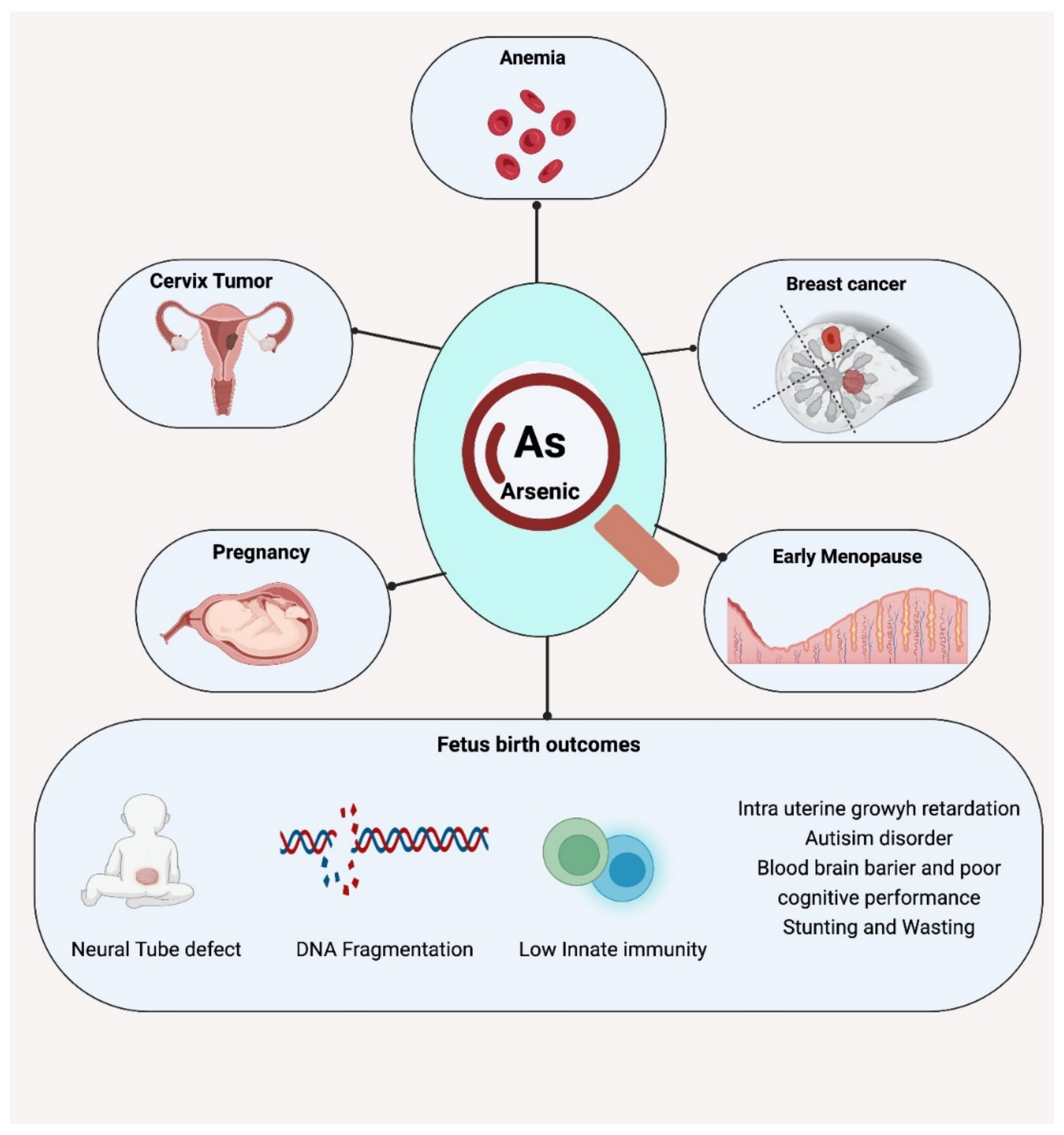
3.2. Effects on Maternal Health
3.3. Effects on Fetal and Neonatal Health
This entry is adapted from the peer-reviewed paper 10.3390/nu14102136
References
- Ezemonye, L.I.; Adebayo, P.O.; Enuneku, A.A.; Tongo, I.; Ogbomida, E. Potential health risk consequences of heavy metal concentrations in surface water, shrimp (Macrobrachium macrobrachion) and fish (Brycinus longipinnis) from Benin River, Nigeria. Toxicol. Rep. 2019, 6, 1–9.
- Hill-Briggs, F.; Adler, N.E.; Berkowitz, S.A.; Chin, M.H.; Gary-Webb, T.L.; Navas-Acien, A.; Thornton, P.L.; Haire-Joshu, D. Social determinants of health and diabetes: A scientific review. Diabetes Care 2021, 44, 258–279.
- Authority, E.F.S.; Arcella, D.; Cascio, C.; Gómez Ruiz, J.Á. Chronic dietary exposure to inorganic arsenic. EFSA J. 2021, 19, e06380.
- Chou, C.-H.; Harper, C. Toxicological Profile for Arsenic. 2007. Available online: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/657856 (accessed on 1 March 2022).
- ATSDR (Agency for Toxic Substances and Disease Registry). Prepared by Clement International Corp., under contract 2000, 205, 88-0608.
- Wang, J.; Hu, W.; Yang, H.; Chen, F.; Shu, Y.; Zhang, G.; Liu, J.; Liu, Y.; Li, H.; Guo, L. Arsenic concentrations, diversity and co-occurrence patterns of bacterial and fungal communities in the feces of mice under sub-chronic arsenic exposure through food. Environ. Int. 2020, 138, 105600.
- Watanabe, T.; Hirano, S. Metabolism of arsenic and its toxicological relevance. Arch. Toxicol. 2013, 87, 969–979.
- Eckstein, M.; Eleazer, R.; Rea, M.; Fondufe-Mittendorf, Y. Epigenomic reprogramming in inorganic arsenic-mediated gene expression patterns during carcinogenesis. Rev. Environ. Health 2017, 32, 93–103.
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cravedi, J.; di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; Farmer, P.; et al. Scientific Opinion on marine biotoxins in shellfish—Palytoxin group. EFSA J. 2009, 7, 1393.
- Signes-Pastor, A.J.; Woodside, J.V.; McMullan, P.; Mullan, K.; Carey, M.; Karagas, M.R.; Meharg, A.A. Levels of infants’ urinary arsenic metabolites related to formula feeding and weaning with rice products exceeding the EU inorganic arsenic standard. PLoS ONE 2017, 12, e0176923.
- Molin, M.; Ulven, S.M.; Meltzer, H.M.; Alexander, J. Arsenic in the human food chain, biotransformation and toxicology–Review focusing on seafood arsenic. J. Trace Elem. Med. Biol. 2015, 31, 249–259.
- Yoshinaga, J.; Narukawa, T. Dietary intake and urinary excretion of methylated arsenicals of Japanese adults consuming marine foods and rice. Food Addit. Contam. Part A 2021, 38, 622–629.
- Tibon, J.; Silva, M.; Sloth, J.J.; Amlund, H.; Sele, V. Speciation analysis of organoarsenic species in marine samples: Method optimization using fractional factorial design and method validation. Anal. Bioanal. Chem. 2021, 413, 3909–3923.
- Taylor, V.F.; Jackson, B.P. Concentrations and speciation of arsenic in New England seaweed species harvested for food and agriculture. Chemosphere 2016, 163, 6–13.
- Sattar, A.; Xie, S.; Hafeez, M.A.; Wang, X.; Hussain, H.I.; Iqbal, Z.; Pan, Y.; Iqbal, M.; Shabbir, M.A.; Yuan, Z. Metabolism and toxicity of arsenicals in mammals. Environ. Toxicol. Pharmacol. 2016, 48, 214–224.
- Wang, D.; Shimoda, Y.; Wang, S.; Wang, Z.; Liu, J.; Liu, X.; Jin, H.; Gao, F.; Tong, J.; Yamanaka, K. Total arsenic and speciation analysis of saliva and urine samples from individuals living in a chronic arsenicosis area in China. Environ. Health Prev. Med. 2017, 22, 45.
- Zhang, Q.; Li, Y.; Liu, J.; Wang, D.; Zheng, Q.; Sun, G. Differences of urinary arsenic metabolites and methylation capacity between individuals with and without skin lesions in Inner Mongolia, Northern China. Int. J. Environ. Res. Public Health 2014, 11, 7319–7332.
- Roggenbeck, B.A.; Banerjee, M.; Leslie, E.M. Cellular arsenic transport pathways in mammals. J. Environ. Sci. 2016, 49, 38–58.
- Moe, B.; Peng, H.; Lu, X.; Chen, B.; Chen, L.W.; Gabos, S.; Li, X.-F.; Le, X.C. Comparative cytotoxicity of fourteen trivalent and pentavalent arsenic species determined using real-time cell sensing. J. Environ. Sci. 2016, 49, 113–124.
- Hubaux, R.; Becker-Santos, D.D.; Enfield, K.S.; Rowbotham, D.; Lam, S.; Lam, W.L.; Martinez, V.D. Molecular features in arsenic-induced lung tumors. Mol. Cancer 2013, 12, 1–11.
- Dheeman, D.S.; Packianathan, C.; Pillai, J.K.; Rosen, B.P. Pathway of human AS3MT arsenic methylation. Chem. Res. Toxicol. 2014, 27, 1979–1989.
- Roggenbeck, B.A.; Leslie, E.M.; Walk, S.T.; Schmidt, E.E. Redox metabolism of ingested arsenic: Integrated activities of microbiome and host on toxicological outcomes. Curr. Opin. Toxicol. 2019, 13, 90–98.
- Flora, S.J. Toxic metals: Health effects, and therapeutic measures. J. Biomed. Ther. Sci. 2014, 1, 48–64.
- Styblo, M.; Serves, S.V.; Cullen, W.R.; Thomas, D.J. Comparative inhibition of yeast glutathione reductase by arsenicals and arsenothiols. Chem. Res. Toxicol. 1997, 10, 27–33.
- Mishra, D.; Mehta, A.; Flora, S.J. Reversal of arsenic-induced hepatic apoptosis with combined administration of DMSA and its analogues in guinea pigs: Role of glutathione and linked enzymes. Chem. Res. Toxicol. 2008, 21, 400–407.
- Chakraborti, D.; Rahman, M.M.; Ahamed, S.; Dutta, R.N.; Pati, S.; Mukherjee, S.C. Arsenic groundwater contamination and its health effects in Patna district (capital of Bihar) in the middle Ganga plain, India. Chemosphere 2016, 152, 520–529.
- Hu, Y.; Xiao, T.; Zhang, A. Associations between and risks of trace elements related to skin and liver damage induced by arsenic from coal burning. Ecotoxicol. Environ. Saf. 2021, 208, 111719.
- Chervona, Y.; Arita, A.; Costa, M. Carcinogenic metals and the epigenome: Understanding the effect of nickel, arsenic, and chromium. Metallomics 2012, 4, 619–627.
- Calatayud, M.; Barrios, J.A.; Vélez, D.; Devesa, V. In vitro study of transporters involved in intestinal absorption of inorganic arsenic. Chem. Res. Toxicol. 2012, 25, 446–453.
- Frediani, J.K.; Naioti, E.A.; Vos, M.B.; Figueroa, J.; Marsit, C.J.; Welsh, J.A. Arsenic exposure and risk of nonalcoholic fatty liver disease (NAFLD) among US adolescents and adults: An association modified by race/ethnicity, NHANES 2005–2014. Environ. Health 2018, 17, 6.
- Bunderson, M.; Coffin, J.D.; Beall, H.D. Arsenic induces peroxynitrite generation and cyclooxygenase-2 protein expression in aortic endothelial cells: Possible role in atherosclerosis. Toxicol. Appl. Pharmacol. 2002, 184, 11–18.
- Chen, Y.; Factor-Litvak, P.; Howe, G.R.; Graziano, J.H.; Brandt-Rauf, P.; Parvez, F.; Van Geen, A.; Ahsan, H. Arsenic exposure from drinking water, dietary intakes of B vitamins and folate, and risk of high blood pressure in Bangladesh: A population-based, cross-sectional study. Am. J. Epidemiol. 2007, 165, 541–552.
- Bustaffa, E.; Stoccoro, A.; Bianchi, F.; Migliore, L. Genotoxic and epigenetic mechanisms in arsenic carcinogenicity. Arch. Toxicol. 2014, 88, 1043–1067.
- Tao, X.; Wang, N.; Qin, W. Gut microbiota and hepatocellular carcinoma. Gastrointest. Tumors 2015, 2, 33–40.
- Lu, K.; Abo, R.P.; Schlieper, K.A.; Graffam, M.E.; Levine, S.; Wishnok, J.S.; Swenberg, J.A.; Tannenbaum, S.R.; Fox, J.G. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: An integrated metagenomics and metabolomics analysis. Environ. Health Perspect. 2014, 122, 284–291.
- Sinha, D.; Roy, M. Antagonistic role of tea against sodium arsenite-induced oxidative DNA damage and inhibition of DNA repair in Swiss albino mice. J. Environ. Pathol. Toxicol. Oncol. 2011, 30, 311–312.
- D’Ippoliti, D.; Santelli, E.; De Sario, M.; Scortichini, M.; Davoli, M.; Michelozzi, P. Arsenic in drinking water and mortality for cancer and chronic diseases in Central Italy, 1990-2010. PLoS ONE 2015, 10, e0138182.
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.; Jayasumana, C.; de Silva, P.M.C. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846.
- Dangleben, N.L.; Skibola, C.F.; Smith, M.T. Arsenic immunotoxicity: A review. Environ. Health 2013, 12, 73.
- Rehman, M.Y.A.; Briedé, J.J.; van Herwijnen, M.; Krauskopf, J.; Jennen, D.G.; Malik, R.N.; Kleinjans, J.C. Integrating SNPs-based genetic risk factor with blood epigenomic response of differentially arsenic-exposed rural subjects reveals disease-associated signaling pathways. Environ. Pollut. 2022, 292, 118279.
- Martinez, V.D.; Vucic, E.A.; Adonis, M.; Gil, L.; Lam, W.L. Arsenic biotransformation as a cancer promoting factor by inducing DNA damage and disruption of repair mechanisms. Mol. Biol. Int. 2011, 2011, 718974.
- Soni, M.; Prakash, C.; Sehwag, S.; Kumar, V. Protective effect of hydroxytyrosol in arsenic-induced mitochondrial dysfunction in rat brain. J. Biochem. Mol. Toxicol. 2017, 31, e21906.
- Akbal, A.; Yılmaz, H.; Tutkun, E. Arsenic exposure associated with decreased bone mineralization in male. Aging Male 2014, 17, 256–258.
- Singh, M.K.; Dwivedi, S.; Yadav, S.S.; Yadav, R.S.; Khattri, S. Anti-diabetic effect of Emblica officinalis (Amla) against arsenic induced metabolic disorder in mice. Indian J. Clin. Biochem. 2020, 35, 179–187.
- Carlson, P.; van Beneden, R.J. Arsenic exposure alters expression of cell cycle and lipid metabolism genes in the liver of adult zebrafish (Danio rerio). Aquat. Toxicol. 2014, 153, 66–72.
- Afolabi, O.K.; Wusu, A.D.; Ogunrinola, O.O.; Abam, E.O.; Babayemi, D.O.; Dosumu, O.; Onunkwor, O.; Balogun, E.; Odukoya, O.O.; Ademuyiwa, O. Arsenic-induced dyslipidemia in male albino rats: Comparison between trivalent and pentavalent inorganic arsenic in drinking water. BMC Pharmacol. Toxicol. 2015, 16, 15.
- Souza, A.; Bastos, D.; Sertorio, M.; Santos, F.; Ervilha, L.; de Oliveira, L.; Machado-Neves, M. Combined effects of arsenic exposure and diabetes on male reproductive functions. Andrology 2019, 7, 730–740.
- Wang, X.; Wu, Y.; Sun, X.; Guo, Q.; Xia, W.; Wu, Y.; Li, J.; Xu, S.; Li, Y. Arsenic exposure and metabolism in relation to blood pressure changes in pregnant women. Ecotoxicol. Environ. Saf. 2021, 222, 112527.
- Faita, F.; Cori, L.; Bianchi, F.; Andreassi, M.G. Arsenic-induced genotoxicity and genetic susceptibility to arsenic-related pathologies. Int. J. Environ. Res. Public Health 2013, 10, 1527–1546.
- Tokar, E.J.; Benbrahim-Tallaa, L.; Ward, J.M.; Lunn, R.; Sams, R.L.; Waalkes, M.P. Cancer in experimental animals exposed to arsenic and arsenic compounds. Crit. Rev. Toxicol. 2010, 40, 912–927.
- Fatoki, J.O.; Badmus, J.A. Arsenic as an environmental and human health antagonist: A review of its toxicity and disease initiation. J. Hazard. Mater. Adv. 2022, 100052.
- Xu, P.; Liu, A.; Li, F.; Tinkov, A.A.; Liu, L.; Zhou, J.-C. Associations between metabolic syndrome and four heavy metals: A systematic review and meta-analysis. Environ. Pollut. 2021, 273, 116480.
- Li, G.; Sun, G.-X.; Williams, P.N.; Nunes, L.; Zhu, Y.-G. Inorganic arsenic in Chinese food and its cancer risk. Environ. Int. 2011, 37, 1219–1225.
- Biswas, R.; Ghosh, P.; Banerjee, N.; Das, J.; Sau, T.; Banerjee, A.; Roy, S.; Ganguly, S.; Chatterjee, M.; Mukherjee, A. Analysis of T-cell proliferation and cytokine secretion in the individuals exposed to arsenic. Hum. Exp. Toxicol. 2008, 27, 381–386.
- Islam, L.N.; Nurun Nabi, A.; Rahman, M.M.; Zahid, M.S.H. Association of respiratory complications and elevated serum immunoglobulins with drinking water arsenic toxicity in human. J. Environ. Sci. Health Part A 2007, 42, 1807–1814.
- Robles-Osorio, M.L.; Sabath-Silva, E.; Sabath, E. Arsenic-mediated nephrotoxicity. Ren. Fail. 2015, 37, 542–547.
- Li, Z.; Piao, F.; Liu, S.; Wang, Y.; Qu, S. Subchronic exposure to arsenic trioxide-induced oxidative DNA damage in kidney tissue of mice. Exp. Toxicol. Pathol. 2010, 62, 543–547.
- Hossain, E.; Ota, A.; Takahashi, M.; Karnan, S.; Damdindorj, L.; Konishi, Y.; Konishi, H.; Hosokawa, Y. Arsenic upregulates the expression of angiotensin II Type I receptor in mouse aortic endothelial cells. Toxicol. Lett. 2013, 220, 70–75.
- Li, L.; Bi, Z.; Wadgaonkar, P.; Lu, Y.; Zhang, Q.; Fu, Y.; Thakur, C.; Wang, L.; Chen, F. Metabolic and Epigenetic Reprogramming in the Arsenic-Induced Cancer Stem Cells. In Proceedings of the Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2019; pp. 10–18.
- Muzaffar, S.; Khan, J.; Srivastava, R.; Gorbatyuk, M.S.; Athar, M. Mechanistic understanding of the toxic effects of arsenic and warfare arsenicals on human health and environment. Cell Biol. Toxicol. 2022, 1–26.
- Xu, L.; Polya, D.A.; Li, Q.; Mondal, D. Association of low-level inorganic arsenic exposure from rice with age-standardized mortality risk of cardiovascular disease (CVD) in England and Wales. Sci. Total Environ. 2020, 743, 140534.
- Kulshrestha, A.; Jarouliya, U.; Prasad, G.; Flora, S.; Bisen, P.S. Arsenic-induced abnormalities in glucose metabolism: Biochemical basis and potential therapeutic and nutritional interventions. World J. Transl. Med. 2014, 3, 96–111.
- Sung, T.-C.; Huang, J.-W.; Guo, H.-R. Association between arsenic exposure and diabetes: A meta-analysis. BioMed Res. Int. 2015, 2015.
- Cárdenas-González, M.; Osorio-Yáñez, C.; Gaspar-Ramírez, O.; Pavković, M.; Ochoa-Martinez, A.; López-Ventura, D.; Medeiros, M.; Barbier, O.; Pérez-Maldonado, I.; Sabbisetti, V. Environmental exposure to arsenic and chromium in children is associated with kidney injury molecule-1. Environ. Res. 2016, 150, 653–662.
- Hsu, L.-I.; Hsieh, F.-I.; Wang, Y.-H.; Lai, T.-S.; Wu, M.-M.; Chen, C.-J.; Chiou, H.-Y.; Hsu, K.-H. Arsenic exposure from drinking water and the incidence of CKD in low to moderate exposed areas of Taiwan: A 14-year prospective study. Am. J. Kidney Dis. 2017, 70, 787–797.
- Li, W.; Wu, L.; Sun, Q.; Yang, Q.; Xue, J.; Shi, M.; Tang, H.; Zhang, J.; Liu, Q. MicroRNA-191 blocking the translocation of GLUT4 is involved in arsenite-induced hepatic insulin resistance through inhibiting the IRS1/AKT pathway. Ecotoxicol. Environ. Saf. 2021, 215, 112130.
- López-Carrillo, L.; Hernández-Ramírez, R.U.; Gandolfi, A.J.; Ornelas-Aguirre, J.M.; Torres-Sánchez, L.; Cebrian, M.E. Arsenic methylation capacity is associated with breast cancer in northern Mexico. Toxicol. Appl. Pharmacol. 2014, 280, 53–59.
- Smith, N.K.; Keltie, E.; Sweeney, E.; Weerasinghe, S.; MacPherson, K.; Kim, J.S. Toenail speciation biomarkers in arsenic-related disease: A feasibility study for investigating the association between arsenic exposure and chronic disease. Ecotoxicol. Environ. Saf. 2022, 232, 113269.
- Díaz-Villaseñor, A.; Burns, A.L.; Hiriart, M.; Cebrián, M.E.; Ostrosky-Wegman, P. Arsenic-induced alteration in the expression of genes related to type 2 diabetes mellitus. Toxicol. Appl. Pharmacol. 2007, 225, 123–133.
- Sarker, M.; Tony, S.R.; Siddique, A.E.; Karim, M.; Haque, N.; Islam, Z.; Islam, M.; Khatun, M.; Islam, J.; Hossain, S. Arsenic secondary methylation capacity is inversely associated with arsenic exposure-related muscle mass reduction. Int. J. Environ. Res. Public Health 2021, 18, 9730.
- Ambrosio, F.; Brown, E.; Stolz, D.; Ferrari, R.; Goodpaster, B.; Deasy, B.; Distefano, G.; Roperti, A.; Cheikhi, A.; Garciafigueroa, Y. Arsenic induces sustained impairment of skeletal muscle and muscle progenitor cell ultrastructure and bioenergetics. Free. Radic. Biol. Med. 2014, 74, 64–73.
- Zhang, C.; Ferrari, R.; Beezhold, K.; Stearns-Reider, K.; D’Amore, A.; Haschak, M.; Stolz, D.; Robbins, P.D.; Barchowsky, A.; Ambrosio, F. Arsenic promotes NF-κB-mediated fibroblast dysfunction and matrix remodeling to impair muscle stem cell function. Stem Cells 2016, 34, 732–742.
- Calatayud, M.; Devesa, V.; Vélez, D. Differential toxicity and gene expression in Caco-2 cells exposed to arsenic species. Toxicol. Lett. 2013, 218, 70–80.
- Calatayud, M.; Gimeno-Alcañiz, J.V.; Devesa, V.; Vélez, D. Proinflammatory effect of trivalent arsenical species in a co-culture of Caco-2 cells and peripheral blood mononuclear cells. Arch. Toxicol. 2015, 89, 555–564.
- Schreibelt, G.; Kooij, G.; Reijerkerk, A.; van Doorn, R.; Gringhuis, S.I.; van der Pol, S.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Piontek, J. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007, 21, 3666–3676.
- Capaldo, C.T.; Nusrat, A. Cytokine regulation of tight junctions. Biochim. Biophys. Acta Biomembr. 2009, 1788, 864–871.
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20.
- Ashraf, S.A.; Elkhalifa, A.E.O.; Ahmad, M.F.; Patel, M.; Adnan, M.; Sulieman, A.M.E. Probiotic Fermented Foods and Health Promotion. In African Fermented Food Products-New Trends; Springer: Berlin/Heildeberg, Germany, 2022; pp. 59–88.
- Dong, X.; Shulzhenko, N.; Lemaitre, J.; Greer, R.L.; Peremyslova, K.; Quamruzzaman, Q.; Rahman, M.; Hasan, O.S.I.; Joya, S.A.; Golam, M. Arsenic exposure and intestinal microbiota in children from Sirajdikhan, Bangladesh. PLoS ONE 2017, 12, e0188487.
- Wu, F.; Yang, L.; Islam, M.T.; Jasmine, F.; Kibriya, M.G.; Nahar, J.; Barmon, B.; Parvez, F.; Sarwar, G.; Ahmed, A. The role of gut microbiome and its interaction with arsenic exposure in carotid intima-media thickness in a Bangladesh population. Environ. Int. 2019, 123, 104–113.
- Yadav, R.S.; Shukla, R.K.; Sankhwar, M.L.; Patel, D.K.; Ansari, R.W.; Pant, A.B.; Islam, F.; Khanna, V.K. Neuroprotective effect of curcumin in arsenic-induced neurotoxicity in rats. Neurotoxicology 2010, 31, 533–539.
- Ramos-Chávez, L.A.; Rendón-López, C.R.; Zepeda, A.; Silva-Adaya, D.; Del Razo, L.M.; Gonsebatt, M.E. Neurological effects of inorganic arsenic exposure: Altered cysteine/glutamate transport, NMDA expression and spatial memory impairment. Front. Cell. Neurosci. 2015, 2015, 21.
- Salmeri, N.; Villanacci, R.; Ottolina, J.; Bartiromo, L.; Cavoretto, P.; Dolci, C.; Lembo, R.; Schimberni, M.; Valsecchi, L.; Viganò, P. Maternal arsenic exposure and gestational diabetes: A systematic review and meta-analysis. Nutrients 2020, 12, 3094.
- Tseng, C.-H. The potential biological mechanisms of arsenic-induced diabetes mellitus. Toxicol. Appl. Pharmacol. 2004, 197, 67–83.
- Seshadri, N.; Jonasson, M.E.; Hunt, K.L.; Xiang, B.; Cooper, S.; Wheeler, M.B.; Dolinsky, V.W.; Doucette, C.A. Uncoupling protein 2 regulates daily rhythms of insulin secretion capacity in MIN6 cells and isolated islets from male mice. Mol. Metab. 2017, 6, 760–769.
- Mostafa, M.G.; Queen, Z.J.; Cherry, N. Histopathology of cervical cancer and arsenic concentration in well water: An ecological analysis. Int. J. Environ. Res. Public Health 2017, 14, 1185.
- Kile, M.L.; Faraj, J.M.; Ronnenberg, A.G.; Quamruzzaman, Q.; Rahman, M.; Mostofa, G.; Afroz, S.; Christiani, D.C. A cross sectional study of anemia and iron deficiency as risk factors for arsenic-induced skin lesions in Bangladeshi women. BMC Public Health 2016, 16, 158.
- Yunus, F.M.; Rahman, M.J.; Alam, M.Z.; Hore, S.K.; Rahman, M. Relationship between arsenic skin lesions and the age of natural menopause. BMC Public Health 2014, 14, 419.
- Surdu, S.; Bloom, M.S.; Neamtiu, I.A.; Pop, C.; Anastasiu, D.; Fitzgerald, E.F.; Gurzau, E.S. Consumption of arsenic-contaminated drinking water and anemia among pregnant and non-pregnant women in northwestern Romania. Environ. Res. 2015, 140, 657–660.
- Breton, C.V.; Houseman, E.A.; Kile, M.L.; Quamruzzaman, Q.; Rahman, M.; Mahiuddin, G.; Christiani, D.C. Gender-specific protective effect of hemoglobin on arsenic-induced skin lesions. Cancer Epidemiol. Prev. Biomark. 2006, 15, 902–907.
- Ahmed, S.; Khoda, S.M.-e.; Rekha, R.S.; Gardner, R.M.; Ameer, S.S.; Moore, S.; Ekström, E.-C.; Vahter, M.; Raqib, R. Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environ. Health Perspect. 2011, 119, 258–264.
- Davey, J.C.; Bodwell, J.E.; Gosse, J.A.; Hamilton, J.W. Arsenic as an endocrine disruptor: Effects of arsenic on estrogen receptor–mediated gene expression in vivo and in cell culture. Toxicol. Sci. 2007, 98, 75–86.
- Chatterjee, A.; Chatterji, U. Arsenic abrogates the estrogen-signaling pathway in the rat uterus. Reprod. Biol. Endocrinol. 2010, 8, 80.
- Aquino, N.B.; Sevigny, M.B.; Sabangan, J.; Louie, M.C. The role of cadmium and nickel in estrogen receptor signaling and breast cancer: Metalloestrogens or not? J. Environ. Sci. Health Part C 2012, 30, 189–224.
- Marciniak, W.; Derkacz, R.; Muszyńska, M.; Baszuk, P.; Gronwald, J.; Huzarski, T.; Cybulski, C.; Jakubowska, A.; Falco, M.; Dębniak, T. Blood arsenic levels and the risk of familial breast cancer in Poland. Int. J. Cancer 2020, 146, 2721–2727.
- Song, L.; Liu, B.; Zhang, L.; Wu, M.; Wang, L.; Cao, Z.; Zhang, B.; Li, Y.; Wang, Y.; Xu, S. Association of prenatal exposure to arsenic with newborn telomere length: Results from a birth cohort study. Environ. Res. 2019, 175, 442–448.
- Pilsner, J.R.; Hall, M.N.; Liu, X.; Ilievski, V.; Slavkovich, V.; Levy, D.; Factor-Litvak, P.; Yunus, M.; Rahman, M.; Graziano, J.H. Influence of prenatal arsenic exposure and newborn sex on global methylation of cord blood DNA. PLoS ONE 2012, 7, e37147.
- Milton, A.H.; Hussain, S.; Akter, S.; Rahman, M.; Mouly, T.A.; Mitchell, K. A review of the effects of chronic arsenic exposure on adverse pregnancy outcomes. Int. J. Environ. Res. Public Health 2017, 14, 556.
- He, Y.; Pan, A.; Hu, F.B.; Ma, X. Folic acid supplementation, birth defects, and adverse pregnancy outcomes in Chinese women: A population-based mega-cohort study. Lancet 2016, 388, S91.
- Mazumdar, M.; Hasan, M.O.S.I.; Hamid, R.; Valeri, L.; Paul, L.; Selhub, J.; Rodrigues, E.G.; Silva, F.; Mia, S.; Mostofa, M.G. Arsenic is associated with reduced effect of folic acid in myelomeningocele prevention: A case control study in Bangladesh. Environ. Health 2015, 14, 34.
- Parajuli, R.P.; Fujiwara, T.; Umezaki, M.; Watanabe, C. Association of cord blood levels of lead, arsenic, and zinc with neurodevelopmental indicators in newborns: A birth cohort study in Chitwan Valley, Nepal. Environ. Res. 2013, 121, 45–51.
- Demir, N.; Başaranoğlu, M.; Huyut, Z.; Değer, İ.; Karaman, K.; Şekeroğlu, M.R.; Tuncer, O. The relationship between mother and infant plasma trace element and heavy metal levels and the risk of neural tube defect in infants. J. Matern. Fetal Neonatal Med. 2019, 32, 1433–1440.
- Tolins, M.; Ruchirawat, M.; Landrigan, P. The developmental neurotoxicity of arsenic: Cognitive and behavioral consequences of early life exposure. Ann. Glob. Health 2014, 80, 303–314.
- Nadeau, K.C.; Li, Z.; Farzan, S.; Koestler, D.; Robbins, D.; Fei, D.L.; Malipatlolla, M.; Maecker, H.; Enelow, R.; Korrick, S. In utero arsenic exposure and fetal immune repertoire in a US pregnancy cohort. Clin. Immunol. 2014, 155, 188–197.
- Farzan, S.F.; Korrick, S.; Li, Z.; Enelow, R.; Gandolfi, A.J.; Madan, J.; Nadeau, K.; Karagas, M.R. In utero arsenic exposure and infant infection in a United States cohort: A prospective study. Environ. Res. 2013, 126, 24–30.
- Jiang, C.-B.; Hsi, H.-C.; Fan, C.-H.; Chien, L.-C. Fetal exposure to environmental neurotoxins in Taiwan. PLoS ONE 2014, 9, e109984.
- Vahter, M. Effects of arsenic on maternal and fetal health. Annu. Rev. Nutr. 2009, 29, 381–399.
- Bae, S.; Kamynina, E.; Farinola, A.F.; Caudill, M.A.; Stover, P.J.; Cassano, P.A.; Berry, R.; Peña-Rosas, J.P. Provision of folic acid for reducing arsenic toxicity in arsenic-exposed children and adults. Cochrane Database Syst. Rev. 2017, CD012649.
- Arroyo, H.A.; Fernández, M.C. Tóxicos ambientales y su efecto sobre el neurodesarrollo. Medicina 2013, 73.
- Yorifuji, T.; Kato, T.; Ohta, H.; Bellinger, D.C.; Matsuoka, K.; Grandjean, P. Neurological and neuropsychological functions in adults with a history of developmental arsenic poisoning from contaminated milk powder. Neurotoxicol. Teratol. 2016, 53, 75–80.
