Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Alcohol use disorder (AUD) can be defined as a chronically relapsing disorder characterized by the compulsion to ingest alcohol, the loss of control in limiting alcohol intake despite adverse health, social, and occupational consequences, and the emergence of a negative emotional state that can involve feelings of anxiety, irritability, and dysphoria when access to alcohol is prevented, reflecting a state of motivational withdrawal.
- addiction
- alcohol
- alcohol use disorder
- brain
- neurotransmitter systems
1. Introduction
Alcohol use disorder (AUD) can be defined as a chronically relapsing disorder characterized by the compulsion to ingest alcohol, the loss of control in limiting alcohol intake despite adverse health, social, and occupational consequences, and the emergence of a negative emotional state that can involve feelings of anxiety, irritability, and dysphoria when access to alcohol is prevented, reflecting a state of motivational withdrawal [1]. The 5th Edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V), which was published in 2013, has integrated the previously used terms of alcohol abuse and alcohol dependence into a single condition referred to as alcohol use disorder (AUD). This is measured on a scale of severity ranging from mild to severe, depending on the number of diagnostic criteria met by the patient. There are many factors that influence a person’s susceptibility to alcohol addiction, including age at the onset of consumption, genetic predispositions including family history of AUD, as well as stress and other environmental and socioeconomic factors.
AUD is a serious health condition, and alcohol in general is considered one of the leading preventable causes of death in the United States [2], where 14.4 million adults (ages 18+) and over 400,000 adolescents (ages 12–17) have experienced AUD [3]. Globally, the harmful use of alcohol causes approximately 5.9% of all deaths annually, and 5.1% of the global burden of disease is attributable to alcohol consumption [4].
Chronic exposure to alcohol has profound effects on multiple systems throughout the human body, including the cardiovascular, gastrointestinal, and nervous systems [5]. For the purposes of this research, effects outside of the nervous system are briefly described here. For example, heavy alcohol consumption significantly increases the risk of hypertension, atherosclerosis as well as all forms of stroke [6][7][8][9][10]. Furthermore, alcohol use leads to liver cirrhosis and a range of liver diseases, from liver fibrosis to alcoholic hepatitis [11][12]. Outside of the liver, chronic alcohol consumption can lead to other types of gastrointestinal diseases, including cancers [13][14] as well as acute and chronic pancreatitis [15][16]. Of note, AUD can also alter gut microbiota, which in turn can result in neuroinflammation [17][18].
While alcohol consumption can lead to serious psychosocial dysfunction as well as increased incidence of violence, intimate partner aggression, and suicide [19][20][21][22], prolonged alcohol use and alcohol addiction can also have long-term consequences on the brain and other body systems. Acute alcohol consumption leads to short-term alterations in neurological function primarily due to its actions on inhibitory neurotransmission. Whereas repeated consumption of alcohol over time leads to long-term changes in the functioning of several key neural circuits, causing a compulsion to consume this substance despite adverse consequences as well as the development of a negative emotional state when access to alcohol is restricted [1]. These alterations, among others, are characteristic of AUD and are also commonly associated with addiction to drugs other than alcohol.
2. AUD and the Brain
2.1. Effect of Alcohol on Neurotransmitter Systems
Alcohol (ethanol) has a simple chemical structure that allows it to freely diffuse across the lipid bilayer of cell membranes. As such, alcohol molecules can directly interact with components of the cell membranes, such as receptors and transporters, as well as with several intracellular molecules and structures, thus impacting multiple cellular processes and functions. In particular, alcohol is able to alter synaptic function by impacting multiple neurotransmitter systems, including 5-HT, DA, GABA, Glu, and ACh (Figure 1). The following paragraphs briefly summarize some of the main effects of alcohol on these neurotransmitter systems. A more detailed overview of how alcohol impacts neurotransmission can be found elsewhere [23][24][25][26].
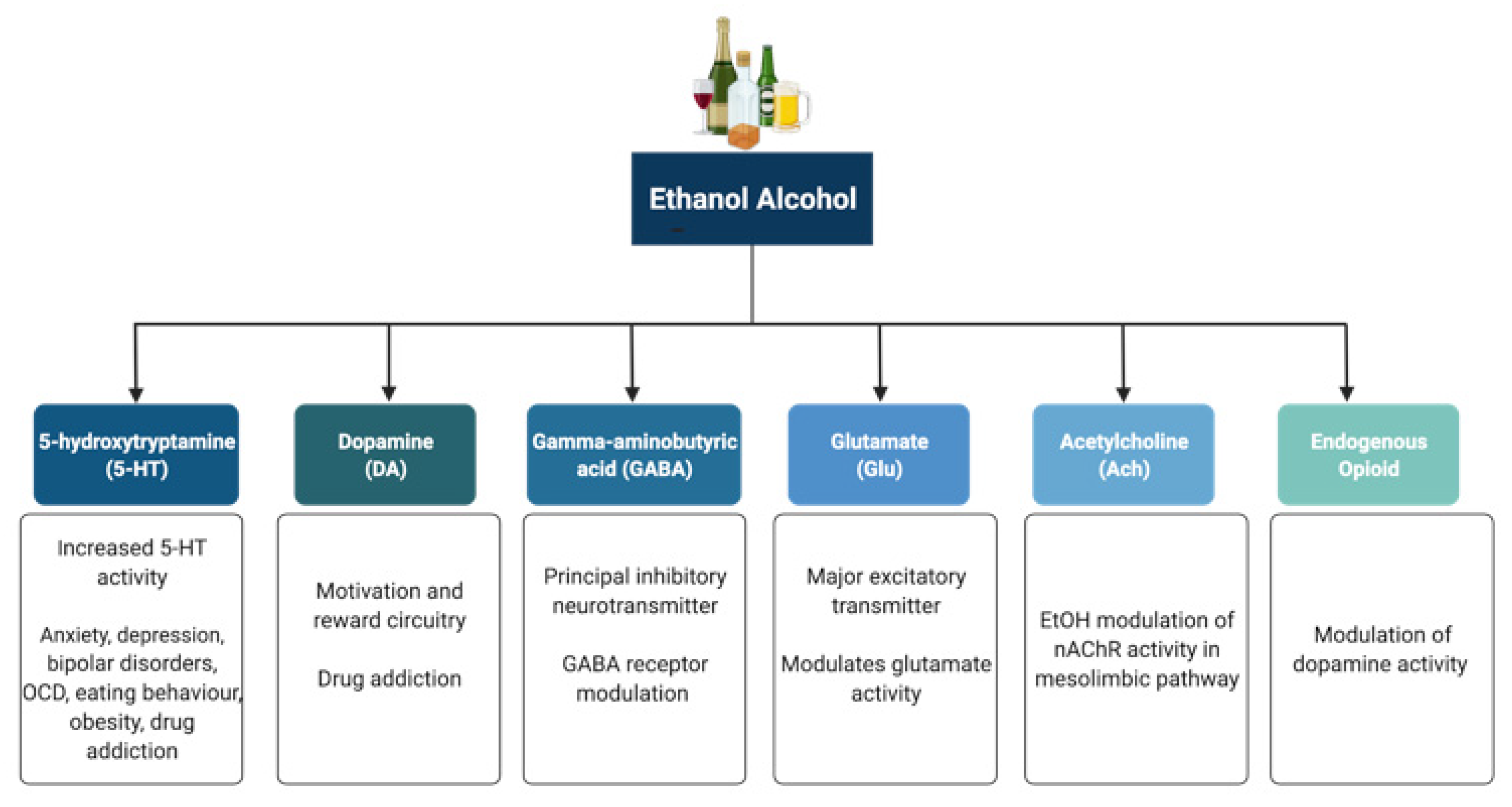
Figure 1. Neurotransmitter systems affected by alcohol (ethanol). Alcohol can interact with multiple neurotransmitter systems in the brain, including the serotonergic (5-HT), dopaminergic (DA), gamma-amynobutyric acid (GABA)-ergic, glutamatergic (Glu), Acetylcholinergic (ACh), and opioid systems, disrupting synaptic transmission and signalling and resulting in the dysregulation of neuronal networks that control reward, motivation, decision making, affect, and the stress response.
5-HT has been implicated in anxiety, depression, bipolar disorders, obsessive compulsive disorders, eating behaviour and obesity, and drug addiction [27][28]. Alcohol has been shown to potentiate the activity of 5-HT3 receptors in murine models using comparable alcohol concentrations to those seen in humans afflicted with AUD [29][30]. Furthermore, genetic variants linked to 5-HT3 receptor sensitivity have been shown to result in an enhanced DAergic reward pathway in humans [31]. In addition, alcohol dependence has been associated with changes in the transcription of the serotonin transporter (5-HTT), which is encoded by the Slc6a4 gene and is responsible for controlling the pattern and magnitude of 5-HT activity [28][32]. Within the Slc6a4 gene, a repeat element of variable length in the 5′ region and a single nucleotide polymorphism (SNP) in the 3′ untranslated region were shown to influence alcohol dependence and severity of drinking as well as response to 5-HT-targeted therapies in AUD patients, respectively [33][34]. Within this scenario, interactive effects of multiple sequence variations at different levels within a specific serotonergic pathway have been proposed to confer greater susceptibility to developing AUD when compared to single variations [35].
DA is known to play a central role in the development of drug addiction, with animal studies suggesting that alcohol administration causes enhanced DAergic neurotransmission within the VTA and a consequent increase in DA levels in the NA [36][37][38]. In AUD, reduced DA receptor sensitivity is thought to decrease motivation for endogenous effectors of the reward circuitry, leading to enhanced compensatory alcohol consumption [39]. Of note, various genetic mutations and polymorphisms that play a role in DAergic neurotransmission have been suggested to contribute to increased vulnerability to alcohol addiction, including the DA receptor D2 Taq1A polymorphism [40][41][42], the DA transporter gene Slc6a3 polymorphism [40][43], and the missense mutation within the catechol-O-methyltransferase (Comt) gene [39][44][45][46]. However, further research is still required to completely elucidate the relationships among genetic factors, DAergic neurotransmission, and the development of AUD.
The endogenous opioid system has important implications for addiction, including modulation of DA release in the NA and of DAergic neurotransmission within the mesolimbic pathway [47]. Polymorphisms of the Oprm1 gene, which encodes the µ-opioid receptor, have been studied in relation to alcohol addiction with mixed results [48][49][50][51][52][53]. Additionally, both the delta and kappa opioid receptors have also been implicated in alcohol addiction [54][55]. Indeed, single nucleotide polymorphisms of Orpk1 and Orpd1 genes may influence behavioural responses to naltrexone [54].
ACh is a neurotransmitter with a wide range of functions both within and outside the central nervous system. SNPs within the cholinergic receptor muscarinic-2 (CHRM2) gene have been associated with predisposition to alcohol and drug dependence and with the development of affective disorders, including major depressive disorder [56][57]. SNPs within the cholinergic receptor nicotinic alpha-5 subunit (CHRNA5) gene have also been associated with alcohol dependence [58].
The eCB system function is also affected by alcohol both acutely and chronically [59], and this system likely plays a complex role in addiction and withdrawal. Acutely, alcohol decreases levels of the eCBs Anandamide (AEA) and 2-arachidonoylglycerol (2-AG) in hippocampal, amygdala, PFC, and cerebellar tissue [60][61][62]. Long-term exposure to alcohol has been documented to reduce both the binding to and expression of the cannabinoid receptor type a (CB1) in the brain [63][64][65][66]. In some cases, these effects can be transient and are not evident after a period of abstinence from alcohol [63][64]. Further research is required in this area in order to better understand how the eCB system is affected by alcohol, as this system has the capacity to influence other neurotransmitter systems responsible for addiction in the brain.
GABA is the principal inhibitory neurotransmitter in the adult human central nervous system. Studies have shown that alcohol allosterically modulates GABAA receptors, and this mechanism may contribute to tolerance, dependence, and withdrawal in AUD [67][68][69]. The sensitivity of GABAA receptors to alcohol has been suggested to be regulated by phosphorylation of the gamma-2 subunit by protein kinase C (PKC) [70][71]. Disruption of PKCɛ, in particular, appears to disrupt voluntary drinking behaviour in mouse models [72][73]. Alcohol has been shown to enhance DAergic neuronal firing rate via decreased firing frequency of GABAergic units within the VTA and NA, thereby reinforcing the effects of alcohol within the pathways involved in reward [74]. In addition, other studies have shown that alcohol increases GABAergic neurotransmission in the cerebellum, hippocampus, and thalamus [75][76][77]. Furthermore, some studies have suggested a potential link between the presence of specific haplotypes within the GABRA2 gene responsible for encoding the α2 subunit of the GABA receptor and susceptibility to developing AUD [78][79][80][81].
Glu is the major excitatory neurotransmitter in the human brain. Acute alcohol exposure generally inhibits Glu neurotransmission, whereas chronic exposure and acute withdrawal have the opposite effect [82]. Alcohol likely affects Glu neurotransmission by altering the function of both metabotropic (mGluRs) and ionotropic (iGluRs) Glu receptors. Upregulation of the metabotropic glutamate receptor 5 (mGluR5)-Homer2-phosphoinositide 3-kinase (PI3K) signalling pathway by binge drinking has been hypothesized to predispose toward a high binge-like alcohol-drinking phenotype [83]. In addition, abnormal hyperactivation of Ras-extracellular signal-regulated kinase (ERK) downstream of mGluR5 results in a hyper-glutamatergic state and has been thought to be a key factor in behaviours associated with addiction [84]. Alcohol drinking was also shown to attenuate the function of D2 DA autoreceptors and group II mGluRs within the posterior VTA [85]. On the other hand, alcohol has inhibitory effects on iGluRs, being capable of inhibiting NMDA receptors [86][87][88]. However, chronic alcohol exposure was shown to increase postsynaptic NMDA receptor function in the rat basolateral amygdala [89]. Of note, the relationship between both the NR2A and NR3A NMDA receptor subunits and susceptibility to addiction has also been investigated, with studies showing a role for these subunits in alcohol dependence and acute NMDA receptor sensitivity to alcohol [90][91]. Variations in the NMDA-dependent α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor trafficking cascade controlling Glu-related excitatory neurotransmission have also been associated with alcohol dependence [92]. Alcohol has also been shown to reduce NMDA receptor expression and function in the NA and to cause deficits in NMDA receptor-dependent long-term depression (LTD) in this brain region after protracted withdrawal [93]. In addition, chronic intermittent ethanol exposure (CIEE) was shown to affect kainate receptors and result in postsynaptic increases in Glu neurotransmission [94] while also increasing the amplitude and frequency of AMPA-receptor-mediated spontaneous excitatory postsynaptic currents in the rat basolateral amygdala [89]. Of note, microinjection of the AMPA-receptor antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX) was capable of attenuating withdrawal-related anxiety-like behaviours, suggesting that increased Glu function may contribute to anxiety during withdrawal from chronic alcohol exposure [89]. A more detailed review of the effects of alcohol on Glu reward circuitry can be found elsewhere [95].
Acute and chronic alcohol exposure has also been shown to affect synaptic plasticity, therefore influencing the efficacy of synaptic transmission at synapses. As explained above, alcohol can directly impact the major excitatory (i.e., glutamatergic) and inhibitory (i.e., GABAergic) neurotransmitter systems within the adult central nervous system, thus effectively contributing to changes in both long-term potentiation (LTP) and LTD, and influencing learning and memory processes [96][97]. Of note, pre-natal alcohol exposure has also been shown to have profound effects on hippocampal synaptic plasticity during development [98].
2.2. Effects of Alcohol on Other Synaptic Targets
Alcohol has been shown to interact both directly and indirectly with additional synaptic and intracellular signalling targets within the brain, and this topic has been reviewed elsewhere [99]. In this section, researchers will present a brief summary of the main effects of alcohol on some of the synaptic and molecular targets within the brain and how these can affect synaptic activity.
Small (SK) and large conductance (BK) Ca2+ and voltage-gated K+ channels have been implicated in alcohol tolerance and adaptive plasticity. Chronic alcohol exposure has been shown to reduce SK channel function in VTA DAergic and CA1 pyramidal neurons and disrupt the SK-channel-NMDA receptor feedback loop, contributing to alcohol-associated adaptive plasticity of glutamatergic synapses [100][101]. Chronic alcohol exposure also leads to enhanced intrinsic excitability and glutamatergic synaptic signalling in lateral orbitofrontal cortical neurons, a mechanism that may contribute to the impairment of behaviours associated with the orbitofrontal cortex in AUD [102], such as anxiety, impulsivity, and aggression [103]. Alcohol has also been shown to interact with BK channels; however, factors such as the level of the activating ligand (intracellular Ca2+), BK subunit composition, post-translational modifications, channel lipid microenvironment, and type of alcohol exposure determine whether or not potentiation or reduction in BK currents occur following alcohol exposure [104]. Alcohol has also been shown to activate G-protein-gated inwardly rectifying potassium (GIRK) channels [105], thereby regulating neuronal excitability and influencing the development of alcohol addiction [106].
In addition to influencing synaptic channels and receptors, there is some evidence that long-term exposure to alcohol may influence synapse structures. Binge alcohol exposure alters scaffolding proteins associated with excitatory synapses [107]. Notably, the morphology of synapses has been shown to be disrupted, and the sizes of dendritic spines are reduced by chronic alcohol exposure in utero, during adolescence, and adulthood in rodent models [108][109][110][111][112][113].
Noteworthy, chronic alcohol use has also been linked to changes in multiple intracellular signalling pathways that can affect synaptic function directly or indirectly. These include alterations in adenosine signalling [114][115], as well as changes in PKC and adenylate cyclase activity [116][117][118].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10051192
References
- Koob, G.F.; Le Moal, M. Drug Abuse: Hedonic Homeostatic Dysregulation. Science 1997, 278, 52–58.
- Centers for Disease Control and Prevention. Report—Alcohol-Attributable Deaths, U.S., By Sex, Excessive Use; Alcohol Related Disease Impact (ARDI) Application; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013. Available online: https://nccd.cdc.gov/DPH_ARDI/Default/Report.aspx?T=AAM&P=f6d7eda7-036e-4553-9968-9b17ffad620e&R=d7a9b303-48e9-4440-bf47-070a4827e1fd&M=8E1C5233-5640-4EE8-9247-1ECA7DA325B9&F=&D (accessed on 12 June 2020).
- Substance Abuse and Mental Health Services Administration (SAMHSA). Results from the 2018 National Survey on Drug Use and Health: Detailed Tables, Sections 1–3; Substance Abuse and Mental Health Services Administration (SAMHSA), Center for Behavioral Health Statistics and Quality (CBHSQ): Rockville, MD, USA, 2018. Available online: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2018R2/NSDUHDetailedTabs2018.pdf (accessed on 12 June 2020).
- World Health Organization. Global Status Report on Alcohol and Health 2014; World Health Organization: Geneve, Switzerland, 2014; pp. 1–392.
- Shield, K.D.; Parry, C.; Rehm, J. Chronic Diseases and Conditions Related to Alcohol Use. Alcohol Res. Curr. Rev. 2014, 35, 155–171.
- Day, E.; Rudd, J.H.F. Alcohol use disorders and the heart. Addiction 2019, 114, 1670–1678.
- Leong, C.; Bolton, J.M.; Ekuma, O.; Prior, H.J.; Singal, D.; Nepon, J.; Konrad, G.; Paillé, M.; Finlayson, G.; Nickel, N. Association of alcohol use disorder on alcohol-related cancers, diabetes, ischemic heart disease and death: A population-based, matched cohort study. Addiction 2021, 117, 368–381.
- Reynolds, K.; Lewis, B.L.; Nolen, J.D.L.; Kinney, G.; Sathya, B.; He, J. Alcohol Consumption and Risk of Stroke. JAMA 2003, 289, 579–588.
- Roerecke, M.; Rehm, J. Chronic heavy drinking and ischaemic heart disease: A systematic review and meta-analysis. Open Hear. 2014, 1, e000135.
- Xi, B.; Veeranki, S.P.; Zhao, M.; Ma, C.; Yan, Y.; Mi, J. Relationship of Alcohol Consumption to All-Cause, Cardiovascular, and Cancer-Related Mortality in U.S. Adults. J. Am. Coll. Cardiol. 2017, 70, 913–922.
- Buchanan, R.; Sinclair, J.M.A. Alcohol use disorder and the liver. Addiction 2020, 116, 1270–1278.
- Crabb, D.W.; Im, G.Y.; Szabo, G.; Mellinger, J.L.; Lucey, M.R. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2020, 71, 306–333.
- Haas, S.L.; Ye, W.; Löhr, M. Alcohol consumption and digestive tract cancer. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 457–467.
- Seitz, H.K.; Maurer, B.; Stickel, F. Alcohol Consumption and Cancer of the Gastrointestinal Tract. Dig. Dis. 2005, 23, 297–303.
- Majumder, S.; Chari, S.T. Chronic pancreatitis. Lancet 2016, 387, 1957–1966.
- Yang, A.L.; Vadhavkar, S.; Singh, G.; Omary, M.B. Epidemiology of Alcohol-Related Liver and Pancreatic Disease in the United States. Arch. Intern. Med. 2008, 168, 649–656.
- Gorky, J.; Schwaber, J. The role of the gut–brain axis in alcohol use disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 65, 234–241.
- Leclercq, S.; Schwarz, M.; Delzenne, N.M.; Stärkel, P.; de Timary, P. Alterations of kynurenine pathway in alcohol use disorder and abstinence: A link with gut microbiota, peripheral inflammation and psychological symptoms. Transl. Psychiatry 2021, 11, 1–9.
- Foran, H.M.; O’Leary, K.D. Alcohol and intimate partner violence: A meta-analytic review. Clin. Psychol. Rev. 2008, 28, 1222–1234.
- Boles, S.M.; Miotto, K. Substance abuse and violence: A review of the literature. Aggress. Violent Behav. 2003, 8, 155–174.
- Sher, L. Alcohol consumption and suicide. QJM 2005, 99, 57–61.
- Butt, P.; Beirness, D.; Gliksman, L.; Paradis, C.; Stockwell, T. Alcohol and Health in Canada: A Summary of Evidence and Guidelines for Low-Risk Drinking; Canadian Centre on Substance Abuse: Ottawa, ON, Canada, 2011; pp. 1–66.
- Faingold, C.; N’Gouemo, P.; Riaz, A. Ethanol and neurotransmitter interactions—From molecular to integrative effects. Prog. Neurobiol. 1998, 55, 509–535.
- Valenzuela, C.F.; Jotty, K. Mini-review: Effects of ethanol on GABAA receptor-mediated neurotransmission in the cere-bellar cortex—Recent advances. Cerebellum 2015, 14, 438–446.
- Valenzuela, C.F.; Puglia, M.P.; Zucca, S. Focus On: Neurotransmitter Systems. Alcohol Res. Health 2011, 34, 106.
- Lovinger, D.M.; Roberto, M. Synaptic Effects Induced by Alcohol. In Behavioral Neurobiology of Alcohol Addiction; Springer: Berlin/Heidelberg, Germany, 2010; pp. 31–86.
- Gingrich, J.A.; Hen, R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology 2001, 155, 1–10.
- Murphy, D.L.; Lerner, A.; Rudnick, G.; Lesch, K.P. Serotonin transporter: Gene, genetic disorders, and pharmacogenetics. Mol. Interv. 2004, 4, 109–123.
- Jun, S.B.; Ikeda, S.R.; Sung, J.E.; Lovinger, D.M. Ethanol induces persistent potentiation of 5-HT3 receptor-stimulated GABA release at synapses on rat hippocampal CA1 neurons. Neuropharmacology 2020, 184, 108415.
- Sung, K.-W.; Engel, S.R.; Allan, A.M.; Lovinger, D.M. 5-HT3 receptor function and potentiation by alcohols in frontal cortex neurons from transgenic mice overexpressing the receptor. Neuropharmacology 2000, 39, 2346–2351.
- Enoch, M.-A.; Gorodetsky, E.; Hodgkinson, C.; Roy, A.; Goldman, D. Functional genetic variants that increase synaptic serotonin and 5-HT3 receptor sensitivity predict alcohol and drug dependence. Mol. Psychiatry 2010, 16, 1139–1146.
- Heinz, A.; Mann, K.; Weinberger, D.R.; Goldman, D. Serotonergic Dysfunction, Negative Mood States, and Response to Alcohol. Alcohol. Clin. Exp. Res. 2001, 25, 487–495.
- McHugh, R.K.; Hofmann, S.G.; Asnaani, A.; Sawyer, A.T.; Otto, M.W. The serotonin transporter gene and risk for alcohol dependence: A meta-analytic review. Drug Alcohol Depend. 2010, 108, 1–6.
- Seneviratne, C.; Huang, W.; Ait-Daoud, N.; Li, M.D.; Johnson, B.A. Characterization of a Functional Polymorphism in the 3′ UTR of SLC6A4 and its Association with Drinking Intensity. Alcohol. Clin. Exp. Res. 2009, 33, 332–339.
- Seneviratne, C.; Franklin, J.; Beckett, K.; Ma, J.Z.; Ait-Daoud, N.; Payne, T.L.; Johnson, B.A.; Li, M.D. Association, interaction, and replication analysis of genes encoding serotonin transporter and 5-HT3 receptor subunits A and B in alcohol dependence. Hum. Genet. 2013, 132, 1165–1176.
- Weiss, F.; Lorang, M.T.; Bloom, F.E.; Koob, G.F. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: Genetic and motivational determinants. J. Pharmacol. Exp. Ther. 1993, 267, 250–258.
- Melendez, R.I.; Rodd-Henricks, Z.A.; Engleman, E.A.; Li, T.-K.; McBride, W.J.; Murphy, J.M. Microdialysis of Dopamine in the Nucleus Accumbens of Alcohol-Preferring (P) Rats during Anticipation and Operant Self-Administration of Ethanol. Alcohol. Clin. Exp. Res. 2002, 26, 318–325.
- Brodie, M.S.; Shefner, S.A.; Dunwiddie, T.V. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990, 508, 65–69.
- Franke, B.; Schellekens, A.F.A.; Ellenbroek, B.; Cools, A.; De Jong, C.A.J.; Buitelaar, J.K.; Verkes, R.-J. Reduced Dopamine Receptor Sensitivity as an Intermediate Phenotype in Alcohol Dependence and the Role of the COMT Val158Met and DRD2 Taq1A Genotypes. Arch. Gen. Psychiatry 2012, 69, 339–348.
- Mignini, F.; Napolioni, V.; Codazzo, C.; Carpi, F.M.; Vitali, M.; Romeo, M.; Ceccanti, M. DRD2/ANKK1 TaqIA and SLC6A3 VNTR polymorphisms in alcohol dependence: Association and gene–gene interaction study in a population of Central Italy. Neurosci. Lett. 2012, 522, 103–107.
- Smith, L.; Watson, M.; Gates, S.; Ball, D.; Foxcroft, D. Meta-Analysis of the Association of the Taq1A Polymorphism with the Risk of Alcohol Dependency: A HuGE Gene-Disease Association Review. Am. J. Epidemiology 2007, 167, 125–138.
- Vasconcelos, A.C.C.G.; Neto, E.D.S.R.; Pinto, G.; Yoshioka, F.K.N.; Motta, F.J.N.; Vasconcelos, D.F.P.; Canalle, R. Association Study of theSLC6A3VNTR (DAT) andDRD2/ANKK1Taq1A Polymorphisms with Alcohol Dependence in a Population from Northeastern Brazil. Alcohol. Clin. Exp. Res. 2015, 39, 205–211.
- Xu, M.; Lin, Z. Genetic influences of dopamine transport gene on alcohol dependence: A pooled analysis of 13 studies with 2483 cases and 1753 controls. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2010, 35, 1255–1260.
- Enoch, M.-A.; Waheed, J.F.; Harris, C.R.; Albaugh, B.; Goldman, D. Sex Differences in the Influence of COMT Val158Met on Alcoholism and Smoking in Plains American Indians. Alcohol. Clin. Exp. Res. 2006, 30, 399–406.
- Tiihonen, J.; Hallikainen, T.; Lachman, H.; Saito, T.; Volavka, J.; Kauhanen, J.; Salonen, J.T.; Ryynänen, O.-P.; Koulu, M.; Karvonen, M.K.; et al. Association between the functional variant of the catechol-O-methyltransferase (COMT) gene and type 1 alcoholism. Mol. Psychiatry 1999, 4, 286–289.
- Foroud, T.; Wetherill, L.F.; Dick, D.M.; Hesselbrock, V.; Nurnberger, J.; Kramer, J.; Tischfield, J.; Schuckit, M.; Bierut, L.J.; Xuei, X.; et al. Lack of Association of Alcohol Dependence and Habitual Smoking with Catechol-O-methyltransferase. Alcohol. Clin. Exp. Res. 2007, 31, 1773–1779.
- Pascale, E.; Lucarelli, M. Alcohol Addiction: A Molecular Biology Perspective. Curr. Med. Chem. 2015, 22, 670–684.
- Bergen, A.W.; Kokoszka, J.; Peterson, R.; Long, J.C.; Virkkunen, M.; Linnoila, M.; Goldman, D. μ opioid receptor gene variants: Lack of association with alcohol dependence. Mol. Psychiatry 1997, 2, 490–494.
- Zhang, Y.; Wang, D.; Johnson, A.D.; Papp, A.C.; Sadée, W. Allelic Expression Imbalance of Human mu Opioid Receptor (OPRM1) Caused by Variant A118G. J. Biol. Chem. 2005, 280, 32618–32624.
- Ramchandani, V.A.; Umhau, J.; Pavon, F.J.; Ruiz-Velasco, V.; Margas, W.; Sun, H.; Damadzic, R.; Eskay, R.; Schoor, M.; Thorsell, A.; et al. A genetic determinant of the striatal dopamine response to alcohol in men. Mol. Psychiatry 2010, 16, 809–817.
- Kranzler, H.R.; Gelernter, J.; O’Malley, S.; Hernandez-Avila, C.A.; Kaufman, D. Association of alcohol or other drug dependence with alleles of the mu opioid receptor gene (OPRM1). Alcohol. Clin. Exp. Res. 1998, 22, 1359–1362.
- Arias, A.; Feinn, R.; Kranzler, H.R. Association of an Asn40Asp (A118G) polymorphism in the μ-opioid receptor gene with substance dependence: A meta-analysis. Drug Alcohol Depend. 2006, 83, 262–268.
- Rouvinen-Lagerström, N.; Lahti, J.; Alho, H.; Kovanen, L.; Aalto, M.; Partonen, T.; Silander, K.; Sinclair, D.; Räikkönen, K.; Eriksson, J.G.; et al. µ-Opioid Receptor Gene (OPRM1) Polymorphism A118G: Lack of Association in Finnish Populations with Alcohol Dependence or Alcohol Consumption. Alcohol Alcohol. 2013, 48, 519–525.
- Ashenhurst, J.R.; Bujarski, S.; Ray, L.A. Delta and kappa opioid receptor polymorphisms influence the effects of naltrexone on subjective responses to alcohol. Pharmacol. Biochem. Behav. 2012, 103, 253–259.
- Poznanski, P.; Lesniak, A.; Korostynski, M.; Szklarczyk, K.; Lazarczyk, M.; Religa, P.; Bujalska-Zadrozny, M.; Sadowski, B.; Sacharczuk, M. Delta-opioid receptor antagonism leads to excessive ethanol consumption in mice with enhanced activity of the endogenous opioid system. Neuropharmacology 2017, 118, 90–101.
- Wang, J.C.; Hinrichs, A.L.; Stock, H.; Budde, J.; Allen, R.; Bertelsen, S.; Kwon, J.M.; Wu, W.; Dick, D.M.; Rice, J.; et al. Evidence of common and specific genetic effects: Association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum. Mol. Genet. 2004, 13, 1903–1911.
- Luo, X.; Kranzler, H.R.; Zuo, L.; Wang, S.; Blumberg, H.; Gelernter, J. CHRM2 gene predisposes to alcohol dependence, drug dependence and affective disorders: Results from an extended case–control structured association study. Hum. Mol. Genet. 2005, 14, 2421–2434.
- Sherva, R.; Kranzler, H.R.; Yu, Y.; Logue, M.W.; Poling, J.; Arias, A.J.; Anton, R.F.; Oslin, D.; Farrer, L.A.; Gelernter, J. Variation in Nicotinic Acetylcholine Receptor Genes is Associated with Multiple Substance Dependence Phenotypes. Neuropsychopharmacology 2010, 35, 1921–1931.
- Wolfe, S. The Synaptic Interactions of Alcohol and the Endogenous Cannabinoid System. Alcohol Res. Curr. Rev. 2022, 42, 3.
- Rubio, M.; De Miguel, R.; Fernández-Ruiz, J.; Gutiérrez-López, M.D.; Carai, M.A.; Ramos, J.A. Effects of a short-term exposure to alcohol in rats on FAAH enzyme and CB1 receptor in different brain areas. Drug Alcohol Depend. 2009, 99, 354–358.
- Rubio, M.; McHugh, D.; Fernández-Ruiz, J.; Bradshaw, H.; Walker, J.M. Short-term exposure to alcohol in rats affects brain levels of anandamide, other N-acylethanolamines and 2-arachidonoyl-glycerol. Neurosci. Lett. 2007, 421, 270–274.
- Ferrer, B.; Bermúdez-Silva, F.J.; Bilbao, A.; Alvarez-Jaimes, L.; Sanchez-Vera, I.; Giuffrida, A.; Serrano, A.; Baixeras, E.; Khaturia, S.; Navarro, M.; et al. Regulation of brain anandamide by acute administration of ethanol. Biochem. J. 2007, 404, 97–104.
- Vinod, K.; Yalamanchili, R.; Xie, S.; Cooper, T.; Hungund, B. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem. Int. 2006, 49, 619–625.
- Ceccarini, J.; Casteels, C.; Koole, M.; Bormans, G.; Van Laere, K. Transient changes in the endocannabinoid system after acute and chronic ethanol exposure and abstinence in the rat: A combined PET and microdialysis study. Eur. J. Pediatr. 2013, 40, 1582–1594.
- Ortiz, S.; Oliva, J.M.; Rial, S.P.; Palomo, T.; Manzanares, J. Chronic ethanol consumption regulates cannabinoid CB1 receptor gene expression in selected regions of rat brain. Alcohol Alcohol. 2004, 39, 88–92.
- Cippitelli, A.; Bilbao, A.; Hansson, A.C.; Del Arco, I.; Sommer, W.; Heilig, M.; Massi, M.; Bermúdez-Silva, F.J.; Navarro, M.; Ciccocioppo, R.; et al. Cannabinoid CB1 receptor antagonism reduces conditioned reinstatement of ethanol-seeking behavior in rats. Eur. J. Neurosci. 2005, 21, 2243–2251.
- Devaud, L.L.; Fritschy, J.-M.; Sieghart, W.; Morrow, A.L. Bidirectional Alterations of GABAA Receptor Subunit Peptide Levels in Rat Cortex during Chronic Ethanol Consumption and Withdrawal. J. Neurochem. 2002, 69, 126–130.
- Mhatre, M.C.; Pena, G.; Sieghart, W.; Ticku, M.K. Antibodies Specific for GABAA Receptor alpha Subunits Reveal that Chronic Alcohol Treatment Down-Regulates alpha-Subunit Expression in Rat Brain Regions. J. Neurochem. 1993, 61, 1620–1625.
- Enoch, M.-A. The role of GABAA receptors in the development of alcoholism. Pharmacol. Biochem. Behav. 2008, 90, 95–104.
- Hodge, C.W.; Mehmert, K.K.; Kelley, S.P.; McMahon, T.; Haywood, A.; Olive, F.; Wang, D.; Sanchez-Perez, A.M.; Messing, R. Supersensitivity to allosteric GABAA receptor modulators and alcohol in mice lacking PKCε. Nat. Neurosci. 1999, 2, 997–1002.
- Qi, Z.-H.; Song, M.; Wallace, M.; Wang, D.; Newton, P.; McMahon, T.; Chou, W.-H.; Zhang, C.; Shokat, K.M.; Messing, R. Protein Kinase C epsilon Regulates gamma-Aminobutyrate Type A Receptor Sensitivity to Ethanol and Benzodiazepines through Phosphorylation of gamma2 Subunits. J. Biol. Chem. 2007, 282, 33052–33063.
- Maiya, R.; McMahon, T.; Wang, D.; Kanter, B.; Gandhi, D.; Chapman, H.L.; Miller, J.; Messing, R.O. Selective chemical genetic inhibition of protein kinase C epsilon reduces ethanol consumption in mice. Neuropharmacology 2016, 107, 40–48.
- Besheer, J.; Lepoutre, V.; Mole, B.; Hodge, C.W. GABAA receptor regulation of voluntary ethanol drinking requires PKCε. Synapse 2006, 60, 411–419.
- Burkhardt, J.M.; Adermark, L. Locus of onset and subpopulation specificity of in vivo ethanol effect in the reciprocal ventral tegmental area–nucleus accumbens circuit. Neurochem. Int. 2014, 76, 122–130.
- Diaz, M.R.; Valenzuela, C.F. Sensitivity of GABAergic Tonic Currents to Acute Ethanol in Cerebellar Granule Neurons is Not Age- or δ Subunit-Dependent in Developing Rats. Alcohol. Clin. Exp. Res. 2016, 40, 83–92.
- Liang, J.; Zhang, N.; Cagetti, E.; Houser, C.R.; Olsen, R.W.; Spigelman, I. Chronic Intermittent Ethanol-Induced Switch of Ethanol Actions from Extrasynaptic to Synaptic Hippocampal GABAA Receptors. J. Neurosci. 2006, 26, 1749–1758.
- Jia, F.; Chandra, D.; Homanics, G.E.; Harrison, N.L. Ethanol Modulates Synaptic and Extrasynaptic GABAA Receptors in the Thalamus. J. Pharmacol. Exp. Ther. 2008, 326, 475–482.
- Edenberg, H.J.; Dick, D.M.; Xuei, X.; Tian, H.; Almasy, L.; Bauer, L.O.; Crowe, R.R.; Goate, A.; Hesselbrock, V.; Jones, K.; et al. Variations in GABRA2, Encoding the α2 Subunit of the GABAA Receptor, Are Associated with Alcohol Dependence and with Brain Oscillations. Am. J. Hum. Genet. 2004, 74, 705–714.
- Agrawal, A.; Edenberg, H.J.; Foroud, T.; Bierut, L.J.; Dunne, G.; Hinrichs, A.L.; Nurnberger, J.I.; Crowe, R.; Kuperman, S.; Schuckit, M.A.; et al. Association of GABRA2 with Drug Dependence in the Collaborative Study of the Genetics of Alcoholism Sample. Behav. Genet. 2006, 36, 640–650.
- Covault, J.; Gelernter, J.; Hesselbrock, V.; Nellissery, M.; Kranzler, H.R. Allelic and haplotypic association ofGABRA2 with alcohol dependence. Am. J. Med Genet. 2004, 129B, 104–109.
- Fehr, C.; Sander, T.; Tadic, A.; Lenzen, K.P.; Anghelescu, I.; Klawe, C.; Dahmen, N.; Schmidt, L.G.; Szegedi, A. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatr. Genet. 2006, 16, 9–17.
- Roberto, M.; Varodayan, F.P. Synaptic targets: Chronic alcohol actions. Neuropharmacology 2017, 122, 85–99.
- Cozzoli, D.K.; Goulding, S.P.; Zhang, P.W.; Xiao, B.; Hu, J.-H.; Ary, A.W.; Obara, I.; Rahn, A.; Abou-Ziab, H.; Tyrrel, B.; et al. Binge Drinking Upregulates Accumbens mGluR5-Homer2-PI3K Signaling: Functional Implications for Alcoholism. J. Neurosci. 2009, 29, 8655–8668.
- Schroeder, J.P.; Spanos, M.; Stevenson, J.R.; Besheer, J.; Salling, M.; Hodge, C.W. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: Blockade by the mGluR5 antagonist MPEP. Neuropharmacology 2008, 55, 546–554.
- Ding, Z.-M.; Ingraham, C.M.; Rodd, Z.A.; McBride, W.J. Alcohol drinking increases the dopamine-stimulating effects of ethanol and reduces D2 auto-receptor and group II metabotropic glutamate receptor function within the posterior ventral tegmental area of alcohol preferring (P) rats. Neuropharmacology 2016, 109, 41–48.
- Dildy, J.E.; Leslie, S.W. Ethanol inhibits NMDA-induced increases in free intracellular Ca2+ in dissociated brain cells. Brain Res. 1989, 499, 383–387.
- Hoffman, P.L.; Rabe, C.S.; Moses, F.; Tabakoff, B. N-Methyl-D-Aspartate Receptors and Ethanol: Inhibition of Calcium Flux and Cyclic GMP Production. J. Neurochem. 1989, 52, 1937–1940.
- Lovinger, D.M.; White, G.; Weight, F.F. Ethanol Inhibits NMDA-Activated Ion Current in Hippocampal Neurons. Science 1989, 243, 1721–1724.
- Läck, A.K.; Diaz, M.; Chappell, A.; DuBois, D.W.; McCool, B.A. Chronic Ethanol and Withdrawal Differentially Modulate Pre- and Postsynaptic Function at Glutamatergic Synapses in Rat Basolateral Amygdala. J. Neurophysiol. 2007, 98, 3185–3196.
- Jin, C.; Smothers, C.T.; Woodward, J.J. Enhanced Ethanol Inhibition of Recombinant N-methyl-D-aspartate Receptors by Magnesium: Role of NR3A Subunits. Alcohol. Clin. Exp. Res. 2008, 32, 1059–1066.
- Schumann, G.; Johann, M.; Frank, J.; Preuss, U.; Dahmen, N.; Laucht, M.; Rietschel, M.; Rujescu, D.; Lourdusamy, A.; Clarke, T.-K.; et al. Systematic Analysis of Glutamatergic Neurotransmission Genes in Alcohol Dependence and Adolescent Risky Drinking Behavior. Arch. Gen. Psychiatry 2008, 65, 826–838.
- Karpyak, V.M.; Geske, J.R.; Colby, C.L.; Mrazek, D.A.; Biernacka, J.M. Genetic variability in the NMDA-dependent AMPA trafficking cascade is associated with alcohol dependence. Addict. Biol. 2011, 17, 798–806.
- Abrahao, K.P.; Ariwodola, O.J.; Butler, T.R.; Rau, A.R.; Skelly, M.J.; Carter, E.; Alexander, N.P.; McCool, B.A.; Souza-Formigoni, M.L.O.; Weiner, J.L. Locomotor sensitization to ethanol impairs NMDA receptor-dependent synaptic plasticity in the nucleus accumbens and increases ethanol self-administration. J. Neurosci. 2013, 33, 4834–4842.
- Läck, A.; Christian, D.; Diaz, M.; McCool, B. Chronic ethanol and withdrawal effects on kainate receptor–mediated excitatory neurotransmission in the rat basolateral amygdala. Alcohol 2009, 43, 25–33.
- Bell, R.L.; Hauser, S.R.; McClintick, J.; Rahman, S.; Edenberg, H.J.; Szumlinski, K.K.; McBride, W.J. Ethanol-Associated Changes in Glutamate Reward Neurocircuitry: A Minireview of Clinical and Preclinical Genetic Findings. Prog. Mol. Biol. Transl. Sci. 2015, 137, 41–85.
- Zorumski, C.F.; Mennerick, S.; Izumi, Y. Acute and chronic effects of ethanol on learning-related synaptic plasticity. Alcohol 2013, 48, 1–17.
- McCool, B.A. Ethanol modulation of synaptic plasticity. Neuropharmacology 2011, 61, 1097–1108.
- Fontaine, C.J.; Patten, A.R.; Sickmann, H.M.; Helfer, J.L.; Christie, B.R. Effects of pre-natal alcohol exposure on hippocampal synaptic plasticity: Sex, age and methodological considerations. Neurosci. Biobehav. Rev. 2016, 64, 12–34.
- Abrahao, K.; Salinas, A.; Lovinger, D.M. Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron 2017, 96, 1223–1238.
- Mulholland, P.J.; Hopf, F.W.; Bukiya, A.N.; Martin, G.E.; Liu, J.; Dopico, A.M.; Bonci, A.; Treistman, S.N.; Chandler, L.J. Sizing up Ethanol-Induced Plasticity: The Role of Small and Large Conductance Calcium-Activated Potassium Channels. Alcohol. Clin. Exp. Res. 2009, 33, 1125–1135.
- Mulholland, P.J.; Becker, H.C.; Woodward, J.J.; Chandler, L.J. Small Conductance Calcium-Activated Potassium Type 2 Channels Regulate Alcohol-Associated Plasticity of Glutamatergic Synapses. Biol. Psychiatry 2011, 69, 625–632.
- Nimitvilai, S.; Lopez, M.F.; Mulholland, P.J.; Woodward, J.J. Chronic Intermittent Ethanol Exposure Enhances the Excitability and Synaptic Plasticity of Lateral Orbitofrontal Cortex Neurons and Induces a Tolerance to the Acute Inhibitory Actions of Ethanol. Neuropsychopharmacology 2015, 41, 1112–1127.
- Kuniishi, H.; Ichisaka, S.; Matsuda, S.; Futora, E.; Harada, R.; Hata, Y. Chronic inactivation of the orbitofrontal cortex increases anxiety-like behavior and impulsive aggression, but decreases depression-like behavior in rats. Front. Behav. Neurosci. 2017, 10, 250.
- Dopico, A.M.; Bukiya, A.N.; Martin, G.E. Ethanol modulation of mammalian BK channels in excitable tissues: Molecular targets and their possible contribution to alcohol-induced altered behavior. Front. Physiol. 2014, 5, 466.
- Bodhinathan, K.; Slesinger, P.A. Molecular mechanism underlying ethanol activation of G-protein–gated inwardly rectifying potassium channels. Proc. Natl. Acad. Sci. USA 2013, 110, 18309–18314.
- Lüscher, C.; Slesinger, P.A. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat. Rev. Neurosci. 2010, 11, 301–315.
- Montesinos, J.; Pascual, M.; Millán-Esteban, D.; Guerri, C. Binge-like ethanol treatment in adolescence impairs autophagy and hinders synaptic maturation: Role of TLR4. Neurosci. Lett. 2018, 682, 85–91.
- Jury, N.J.; Pollack, G.A.; Ward, M.J.; Bezek, J.L.; Ng, A.J.; Pinard, C.R.; Bergstrom, H.; Holmes, A. Chronic Ethanol during Adolescence Impacts Corticolimbic Dendritic Spines and Behavior. Alcohol. Clin. Exp. Res. 2017, 41, 1298–1308.
- Lee, K.; Dunwiddie, T.; Deitrich, R.; Lynch, G.; Hoffer, B. Chronic ethanol consumption and hippocampal neuron dendritic spines: A morphometric and physiological analysis. Exp. Neurol. 1981, 71, 541–549.
- Romero, A.M.; Renau-Piqueras, J.; Marin, M.P.; Timoneda, J.; Berciano, M.T.; Lafarga, M.; Esteban-Pretel, G. Chronic Alcohol Alters Dendritic Spine Development in Neurons in Primary Culture. Neurotox. Res. 2013, 24, 532–548.
- Inomata, K.; Nasu, F.; Tanaka, H. Decreased density of synaptic formation in the frontal cortex of neonatal rats exposed to ethanol in utero. Int. J. Dev. Neurosci. 1987, 5, 455–457.
- Uys, J.D.; McGuier, N.S.; Gass, J.T.; Griffin, W.C.; Ball, L.; Mulholland, P.J. Chronic intermittent ethanol exposure and withdrawal leads to adaptations in nucleus accumbens core postsynaptic density proteome and dendritic spines. Addict. Biol. 2015, 21, 560–574.
- Chandler, L.J.; Carpenter-Hyland, E.; Hendricson, A.W.; Maldve, R.E.; Morrisett, R.A.; Zhou, F.C.; Sari, Y.; Bell, R.; Szumlinski, K.K. Structural and Functional Modifications in Glutamateric Synapses Following Prolonged Ethanol Exposure. Alcohol. Clin. Exp. Res. 2006, 30, 368–376.
- Sharma, R.; Engemann, S.C.; Sahota, P.; Thakkar, M.M. Effects of Ethanol on Extracellular Levels of Adenosine in the Basal Forebrain: An In Vivo Microdialysis Study in Freely Behaving Rats. Alcohol. Clin. Exp. Res. 2010, 34, 813–818.
- Hughes, V.; Richardson, M.J.E.; Wall, M.J. Acute ethanol exposure has bidirectional actions on the endogenous neuromodulator adenosine in rat hippocampus. J. Cereb. Blood Flow Metab. 2018, 175, 1471–1485.
- Pany, S.; Das, J. Alcohol binding in the C1 (C1A+C1B) domain of protein kinase C epsilon. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2015, 1850, 2368–2376.
- Ron, D.; Barak, S. Molecular mechanisms underlying alcohol-drinking behaviours. Nat. Rev. Neurosci. 2016, 17, 576–591.
- Yoshimura, M.; Pearson, S.; Kadota, Y.; Gonzalez, C.E. Identification of Ethanol Responsive Domains of Adenylyl Cyclase. Alcohol. Clin. Exp. Res. 2006, 30, 1824–1832.
This entry is offline, you can click here to edit this entry!
