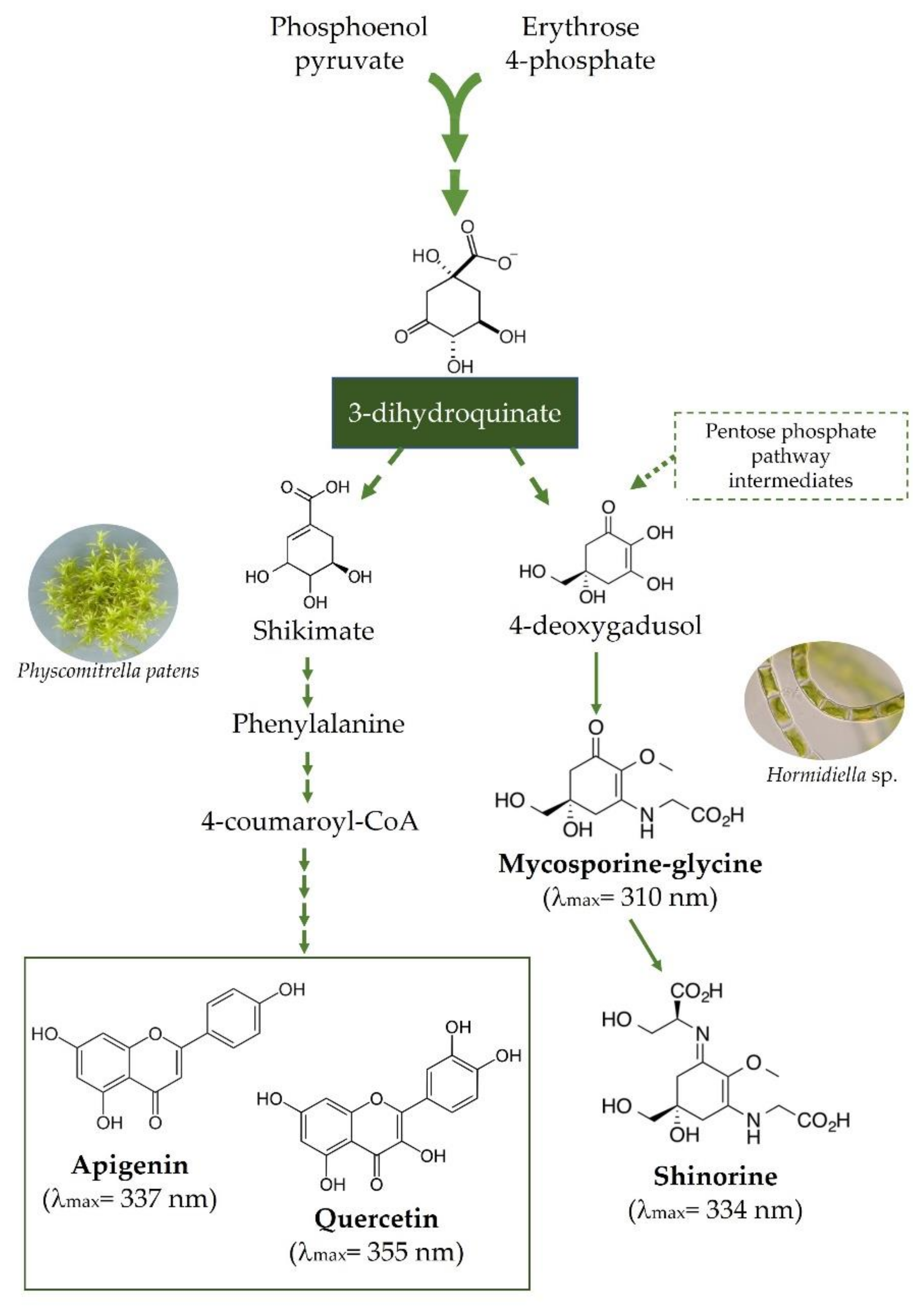
Plants evolved an impressive arsenal of specialized metabolites to cope with the novel environmental pressures imposed by the terrestrial habitat when moving from water. Flavonoids are maybe the most important specilized metabolites that show multifarious roles in the sucess of plant terrestrialization. These compounds modulated auxin transport and signaling and promoted the symbiosis between plants and fungi (e.g., arbuscular mycorrhizal, AM), a central event for the conquest of land by plants. AM improved the ability of early plants to take up nutrients and water from highly impoverished soils. Therefore, flavonoids were essential to plant development in the “new world” scarce of water and nutrients.
- arbuscular mycorrhizal
- auxin
- drought
- nutrient deficiency
- flavonoids
1. Introduction
2. A “Revolutionary” Molecular Innovation: The Rise of Flavonoid Metabolism during Water-to-Land Transition of Plants
3. Arbuscular Mycorrhizal Associations: A Central Event in Plant Terrestrialization Aided by Flavonoids
4. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/ijms23095284
References
- Wagner, A. The molecular origins of evolutionary innovations. Trends Genet. 2011, 27, 397–410.
- Delaux, P.M.; Nanda, A.K.; Mathé, C.; Sejalon-Delmas, N.; Dunand, C. Molecular and biochemical aspects of plant terrestrialization. Perspec. Plant Ecol. Evol. Syst. 2012, 14, 49–59.
- Bowles, A.M.; Bechtold, U.; Paps, J. The origin of land plants is rooted in two bursts of genomic novelty. Curr. Biol. 2020, 30, 530–536.
- De Vries, J.; de Vries, S.; Slamovits, C.H.; Rose, L.E.; Archibald, J.M. How embryophytic is the biosynthesis of phenylpropanoids and their derivatives in streptophyte algae? Plant Cell Physiol. 2017, 58, 934–945.
- Han, X.; Chang, X.; Zhang, Z.; Chen, H.; He, H.; Zhong, B.; Deng, X.W. Origin and evolution of core components responsible for monitoring light environment changes during plant terrestrialization. Mol. Plant 2019, 12, 847–862.
- Buschmann, H.; Holzinger, A. Understanding the algae to land plant transition. J. Exp. Bot. 2020, 71, 3241–3246.
- Cheng, S.; Xian, W.; Fu, Y.; Marin, B.; Keller, J.; Wu, T.; Sun, W.; Li, X.; Xu, Y.; Zhang, Y.; et al. Genomes of subaerial Zygnematophyceae provide insights into land plant evolution. Cell 2019, 179, 1057–1067.
- Donoghue, P.; Paps, J. Plant evolution: Assembling land plants. Curr. Biol. 2020, 30, R81–R83.
- Fürst-Jansen, J.M.; de Vries, S.; de Vries, J. Evo-physio: On stress responses and the earliest land plants. J. Exp. Bot. 2020, 71, 3254–3269.
- Harholt, J.; Moestrup, Ø.; Ulvskov, P. Why plants were terrestrial from the beginning. Trends Plant Sci. 2016, 21, 96–101.
- Moody, L.A. Three-dimensional growth: A developmental innovation that facilitated plant terrestrialization. J. Plant Res. 2020, 133, 283–290.
- Blázquez, M.A.; Nelson, D.C.; Weijers, D. Evolution of plant hormone response pathways. Ann. Rev. Plant Biol. 2020, 71, 327–353.
- Pires, N.D.; Dolan, L. Morphological evolution in land plants: New designs with old genes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 508–518.
- Weng, J.K. The evolutionary paths towards complexity: A metabolic perspective. New Phytol. 2014, 201, 1141–1149.
- Nishiyama, T.; Sakayama, H.; De Vries, J.; Buschmann, H.; Saint-Marcoux, D.; Ullrich, K.K.; Rensing, S.A. The Chara genome: Secondary complexity and implications for plant terrestrialization. Cell 2018, 174, 448–464.
- Leslie, A.B.; Simpson, C.; Mander, L. Reproductive innovations and pulsed rise in plant complexity. Science 2021, 373, 1368–1372.
- Moody, L.A. The 2D to 3D growth transition in the moss Physcomitrella patens. Curr. Opin. Plant Biol. 2019, 47, 88–95.
- Hata, Y.; Kyozuka, J. Fundamental mechanisms of the stem cell regulation in land plants: Lesson from shoot apical cells in bryophytes. Plant Mol. Biol. 2021, 107, 213–225.
- Michniewicz, M.; Zago, M.K.; Abas, L.; Weijers, D.; Schweighofer, A.; Meskiene, I.; Heisler, M.G.; Ohno, C.; Zhang, J.; Huang, F.; et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 2007, 130, 1044–1056.
- Viaene, T.; Landberg, K.; Thelander, M.; Medvecka, E.; Pederson, E.; Feraru, E.; Friml, J. Directional auxin transport mechanisms in early diverging land plants. Curr. Biol. 2014, 24, 2786–2791.
- Vosolsobě, S.; Skokan, R.; Petrášek, J. The evolutionary origins of auxin transport: What we know and what we need to know. J. Exp. Bot. 2020, 71, 3287–3295.
- Bennett, T. PIN proteins and the evolution of plant development. Trends Plant Sci. 2015, 20, 498–507.
- Morffy, N.; Strader, L.C. Old Town Roads: Routes of auxin biosynthesis across kingdoms. Curr. Opin. Plant Biol. 2020, 55, 21–27.
- Komatsu, K.; Suzuki, N.; Kuwamura, M.; Nishikawa, Y.; Nakatani, M.; Ohtawa, H.; Sakata, Y. Group A PP2Cs evolved in land plants as key regulators of intrinsic desiccation tolerance. Nat. Commun. 2013, 4, 2219.
- Stevenson, S.R.; Kamisugi, Y.; Trinh, C.H.; Schmutz, J.; Jenkins, J.W.; Grimwood, J.; Cuming, A.C. Genetic analysis of Physcomitrella patens identifies ABSCISIC ACID NON-RESPONSIVE, a regulator of ABA responses unique to basal land plants and required for desiccation tolerance. Plant Cell 2016, 28, 1310–1327.
- Sun, Y.; Pri-Tal, O.; Michaeli, D.; Mosquna, A. Evolution of abscisic acid signaling module and its perception. Front. Plant Sci. 2020, 11, 934.
- Umezawa, T.; Nakashima, K.; Miyakawa, T.; Kuromori, T.; Tanokura, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular basis of the core regulatory network in ABA responses: Sensing, signaling and transport. Plant Cell Physiol. 2010, 51, 1821–1839.
- Cominelli, E.; Galbiati, M.; Vavasseur, A.; Conti, L.; Sala, T.; Vuylsteke, M.; Tonelli, C. A guard-cell-specific MYB transcription factor regulates stomatal movements and plant drought tolerance. Curr. Biol. 2005, 15, 1196–1200.
- De Zelicourt, A.; Colcombet, J.; Hirt, H. The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci. 2016, 21, 677–685.
- Brunetti, C.; Sebastiani, F.; Tattini, M. ABA, flavonols, and the evolvability of land plants. Plant Sci. 2019, 280, 448–454.
- Weng, J.K.; Chapple, C. The origin and evolution of lignin biosynthesis. New Phytol. 2010, 187, 273–285.
- Milo, R.; Last, R.L. Achieving diversity in the face of constraints: Lessons from metabolism. Science 2012, 336, 1663–1667.
- Mutwil, M. Computational approaches to unravel the pathways and evolution of specialized metabolism. Curr. Opin. Plant Biol. 2020, 55, 38–46.
- Weng, J.K.; Lynch, J.H.; Matos, J.O.; Dudareva, N. Adaptive mechanisms of plant specialized metabolism connecting chemistry to function. Nat. Chem. Biol. 2021, 17, 1037–1045.
- Jones, C.G.; Firn, R.D. On the evolution of plant secondary chemical diversity. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1991, 333, 273–280.
- Neilson, E.H.; Goodger, J.Q.; Woodrow, I.E.; Møller, B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2013, 18, 250–258.
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are flavonoids effective antioxidants in plants? Twenty years of our investigation. Antioxidants 2020, 9, 1098.
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209.
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52.
- Hu, L.; Wu, Z.; Robert, C.A.; Ouyang, X.; Züst, T.; Mestrot, A.; Erb, M. Soil chemistry determines whether defensive plant secondary metabolites promote or suppress herbivore growth. Proc. Natl. Acad. Sci. USA 2021, 118, e2109602118.
- Davies, K.M.; Jibran, R.; Zhou, Y.; Albert, N.W.; Brummell, D.A.; Jordan, B.R.; Bowman, J.L.; Schwinn, K.E. The evolution of flavonoid biosynthesis: A bryophyte perspective. Front. Plant Sci. 2020, 11, 7.
- Davies, K.M.; Jibran, R.; Albert, N.W.; Zhou, Y.; Schwinn, K.E. Conservation and divergence between bryophytes and angiosperms in the biosynthesis and regulation of flavonoid production. In Recent Advances Polyphenols Research; Reed, J.D., Freitas, V.A.P., Quideau, S., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; Volume 7, pp. 227–263.
- Saijo, Y.; Loo, E.P.I. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020, 225, 87–104.
- Wen, W.; Alseekh, S.; Fernie, A.R. Conservation and diversification of flavonoid metabolism in the plant kingdom. Curr. Opin. Plant Biol. 2020, 55, 100–108.
- Böttner, L.; Grabe, V.; Gablenz, S.; Böhme, N.; Appenroth, K.J.; Gershenzon, J.; Huber, M. Differential localization of flavonoid glucosides in an aquatic plant implicates different functions under abiotic stress. Plant Cell Environ. 2021, 44, 900–914.
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19.
- Tian, B.; Pei, Y.; Huang, W.; Ding, J.; Siemann, E. Increasing flavonoid concentrations in root exudates enhance associations between arbuscular mycorrhizal fungi and an invasive plant. ISME J. 2021, 15, 1919–1930.
- Pollastri, S.; Tattini, M. Flavonols: Old compounds for old roles. Ann. Bot. 2011, 108, 1225–1233.
- Remias, D.; Schwaiger, S.; Aigner, S.; Leya, T.; Stuppner, H.; Lütz, C. Characterization of an UV- and VIS-absorbing, purpurogallin-derived secondary pigment new to algae and highly abundant in Mesotaenium berggrenii (Z ygnematophyceae, Chlorophyta), an extremophyte living on glaciers. FEMS Microbiol. Ecol. 2012, 79, 638–648.
- De Vries, J.; Archibald, J.M. Plant evolution: Landmarks on the path to terrestrial life. New Phytol. 2018, 217, 1428–1434.
- Singh, D.K.; Pathak, J.; Pandey, A.; Singh, V.; Ahmed, H.; Kumar, D.; Sinha, R.P. Ultraviolet-screening compound mycosporine-like amino acids in cyanobacteria: Biosynthesis, functions, and applications. In Advances in Cyanobacterial Biology; Singh, P.K., Kumar, A., Singh, V.K., Shrivastava, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 219–233.
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (MAAs): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63.
- Hartmann, A.; Glaser, K.; Holzinger, A.; Ganzera, M.; Karsten, U. Klebsormidin A and B, two new UV-sunscreen compounds in green microalgal interfilum and Klebsormidium Species (Streptophyta) from terrestrial habitats. Front. Microbiol. 2020, 11, 499.
- Bhatia, S.; Garg, A.; Sharma, K.; Kumar, S.; Sharma, A.; Purohit, A.P. Mycosporine and mycosporine-like amino acids: A paramount tool against ultraviolet irradiation. Pharmacogn. Rev. 2011, 5, 138.
- Lüttge, U. Terrestrialization: The Conquest of Dry Land by Plants. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–25.
- Holzinger, A.; Pichrtová, M. Abiotic stress tolerance of charophyte green algae: New challenges for omics techniques. Front. Plant Sci. 2016, 7, 678.
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446.
- Løvdal, T.; Olsen, K.M.; Slimestad, R.; Verheul, M.; Lillo, C. Synergetic effects of nitrogen depletion, temperature, and light on the content of phenolic compounds and gene expression in leaves of tomato. Phytochemistry 2010, 71, 605–613.
- Wang, Y.; Cheng, X.; Yang, T.; Su, Y.; Lin, S.; Zhang, S.; Zhang, Z. Nitrogen-Regulated Theanine and Flavonoid Biosynthesis in Tea Plant Roots: Protein-Level Regulation Revealed by Multiomics Analyses. J. Agric. Food Chem. 2021, 69, 10002–10016.
- Lu, Y.; Chen, Q.; Bu, Y.; Luo, R.; Hao, S.; Zhang, J.; Yao, Y. Flavonoid accumulation plays an important role in the rust resistance of Malus plant leaves. Front. Plant Sci. 2017, 8, 1286.
- Hernández, I.; Van Breusegem, F. Opinion on the possible role of flavonoids as energy escape valves: Novel tools for nature’s Swiss army knife? Plant Sci. 2010, 179, 297–301.
- Ferreyra, M.L.F.; Serra, P.; Casati, P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol. Plant 2021, 173, 736–749.
- Becker, B.; Marin, B. Streptophyte algae and the origin of embryophytes. Ann. Bot. 2009, 103, 999–1004.
- Cockell, C.S.; Knowland, J. Ultraviolet radiation screening compounds. Biol. Rev. 1999, 74, 311–345.
- Rensing, S.A. Great moments in evolution: The conquest of land by plants. Curr. Opin. Plant Biol. 2018, 42, 49–54.
- Rensing, S.A. How plants conquered land. Cell 2020, 181, 964–966.
- Berbee, M.L.; Strullu-Derrien, C.; Delaux, P.M.; Strother, P.K.; Kenrick, P.; Selosse, M.A.; Taylor, J.W. Genomic and fossil windows into the secret lives of the most ancient fungi. Nat. Rev. Microbiol. 2020, 18, 717–730.
- Laliberté, E.; Grace, J.B.; Huston, M.A.; Lambers, H.; Teste, F.P.; Turner, B.L.; Wardle, D.A. How does pedogenesis drive plant diversity? Trends Ecol. Evol. 2013, 28, 331–340.
- Zemunik, G.; Turner, B.L.; Lambers, H.; Laliberté, E. Increasing plant species diversity and extreme species turnover accompany declining soil fertility along a long-term chronosequence in a biodiversity hotspot. J. Ecol. 2016, 104, 792–805.
- Dievart, A.; Gottin, C.; Périn, C.; Ranwez, V.; Chantret, N. Origin and diversity of plant receptor-like kinases. Ann. Rev. Plant Biol. 2020, 71, 131–156.
- Montero, H.; Lee, T.; Pucker, B.; Ferreras-Garrucho, G.; Oldroyd, G.; Brockington, S.F.; Paszkowski, U. A mycorrhiza-associated receptor-like kinase with an ancient origin in the green lineage. Proc. Natl. Acad. Sci. USA 2021, 118, e2105281118.
- Delaux, P.M.; Radhakrishnan, G.V.; Jayaraman, D.; Cheema, J.; Malbreil, M.; Volkening, J.D.; Ané, J.M. Algal ancestor of land plants was preadapted for symbiosis. Proc. Natl. Acad. Sci. USA 2015, 112, 13390–13395.
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775.
- Wang, B.; Yeun, L.H.; Xue, J.Y.; Liu, Y.; Ané, J.M.; Qiu, Y.L. Presence of three mycorrhizal genes in the common ancestor of land plants suggests a key role of mycorrhizas in the colonization of land by plants. New Phytol. 2010, 186, 514–525.
- Vigneron, N.; Radhakrishnan, G.V.; Delaux, P.M. What have we learnt from studying the evolution of the arbuscular mycorrhizal symbiosis? Curr. Opin. Plant Biol. 2018, 44, 49–56.
- Bonfante, P.; Genre, A. Plants and arbuscular mycorrhizal fungi: An evolutionary-developmental perspective. Trends Plant Sci. 2008, 13, 492–498.
- Field, K.J.; Pressel, S.; Duckett, J.G.; Rimington, W.R.; Bidartondo, M.I. Symbiotic options for the conquest of land. Trends Ecol. Evol. 2015, 30, 477–486.
- Hoysted, G.A.; Kowal, J.; Jacob, A.; Rimington, W.R.; Duckett, J.G.; Pressel, S.; Bidartondo, M.I. A mycorrhizal revolution. Curr. Opin. Plant Biol. 2018, 44, 1–6.
- Rich, M.K.; Vigneron, N.; Libourel, C.; Keller, J.; Xue, L.; Hajheidari, M.; Delaux, P.M. Lipid exchanges drove the evolution of mutualism during plant terrestrialization. Science 2021, 372, 864–868.
- Field, K.J.; Bidartondo, M.I.; Rimington, W.R.; Hoysted, G.A.; Beerling, D.; Cameron, D.D.; Pressel, S. Functional complementarity of ancient plant–fungal mutualisms: Contrasting nitrogen, phosphorus and carbon exchanges between Mucoromycotina and Glomeromycotina fungal symbionts of liverworts. New Phytol. 2019, 223, 908–921.
- Rimington, W.R.; Duckett, J.G.; Field, K.J.; Bidartondo, M.I.; Pressel, S. The distribution and evolution of fungal symbioses in ancient lineages of land plants. Mycorrhiza 2020, 30, 23–49.
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660.
- Rimington, W.R.; Pressel, S.; Duckett, J.G.; Field, K.J.; Read, D.J.; Bidartondo, M.I. Ancient plants with ancient fungi: Liverworts associate with early-diverging arbuscular mycorrhizal fungi. Proc. R. Soc. B 2018, 285, 20181600.
- MacLean, A.M.; Bravo, A.; Harrison, M.J. Plant signaling and metabolic pathways enabling arbuscular mycorrhizal symbiosis. Plant Cell 2017, 29, 2319–2335.
- Cosme, M.; Fernández, I.; Van der Heijden, M.G.; Pieterse, C.M. Non-mycorrhizal plants: The exceptions that prove the rule. Trends Plant Sci. 2018, 23, 577–587.
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363.
- Martin, F.M.; Uroz, S.; Barker, D.G. Ancestral alliances: Plant mutualistic symbioses with fungi and bacteria. Science 2017, 356, eaad4501.
- Fusconi, A. Regulation of root morphogenesis in arbuscular mycorrhizae: What role do fungal exudates, phosphate, sugars and hormones play in lateral root formation? Ann. Bot. 2014, 113, 19–33.
- Etemadi, M.; Gutjahr, C.; Couzigou, J.M.; Zouine, M.; Lauressergues, D.; Timmers, A.; Combier, J.P. Auxin perception is required for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Physiol. 2014, 166, 281–292.
- Chen, X.; Chen, J.; Liao, D.; Ye, H.; Li, C.; Luo, Z.; Xu, G. Auxin-mediated regulation of arbuscular mycorrhizal symbiosis: A role of SlGH3. 4 in tomato. Plant Cell Environ. 2021, 45, 955–968.
- Hanlon, M.T.; Coenen, C. Genetic evidence for auxin involvement in arbuscular mycorrhiza initiation. New Phytol. 2011, 189, 701–709.
- Foo, E.; Ross, J.J.; Jones, W.T.; Reid, J.B. Plant hormones in arbuscular mycorrhizal symbioses: An emerging role for gibberellins. Ann. Bot. 2013, 111, 769–779.
- Ludwig-Müller, J.; Güther, M. Auxins as signals in arbuscular mycorrhiza formation. Plant Sign. Behav. 2007, 2, 194–196.
- Peer, W.A.; Murphy, A.S. Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci. 2007, 12, 556–563.
- Zhang, J.; Peer, W.A. Auxin homeostasis: The DAO of catabolism. J. Exp. Bot. 2017, 68, 3145–3154.
- Gianinazzi-Pearson, V.; Branzanti, B.; Gianinazzi, S. In vitro enhancement of spore germination and early hyphal growth of a vesicular-arbuscular mycorrhizal fungus by host root exudates and plant flavonoids. Symbiosis 1989, 7, 243–255.
- Akiyama, K.; Matsuoka, H.; Hayashi, H. Isolation and identification of a phosphate deficiency-induced C-glycosyl flavonoid that stimulates arbuscular mycorrhiza formation in melon roots. Mol. Plant-Microbe Inter. 2002, 15, 334–340.
- Cesco, S.; Neumann, G.; Tomasi, N.; Pinton, R.; Weisskopf, L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 2010, 329, 1–25.
- Salloum, M.S.; Menduni, M.F.; Luna, C.M. A differential capacity of arbuscular mycorrhizal fungal colonization under well-watered conditions and its relationship with drought stress mitigation in unimproved vs. improved soybean genotypes. Botany 2018, 96, 135–144.
- Dong, W.; Song, Y. The Significance of Flavonoids in the Process of Biological Nitrogen Fixation. Int. J. Mol. Sci. 2020, 21, 5926.
- Pei, Y.; Siemann, E.; Tian, B.; Ding, J. Root of an invasive flavonoids are related to enhanced AMF colonization tree. AoB Plants 2020, 12, plaa002.
- Subramanian, S.; Stacey, G.; Yu, O. Distinct, crucial roles of flavonoids during legume nodulation. Trends Plant Sci. 2007, 12, 282–285.
- Stafford, H.A. Roles of flavonoids in symbiotic and defense functions in legume roots. Bot. Rev. 1997, 63, 27–39.
- Ng, J.L.P.; Hassan, S.; Truong, T.T.; Hocart, C.H.; Laffont, C.; Frugier, F.; Mathesius, U. Flavonoids and auxin transport inhibitors rescue symbiotic nodulation in the Medicago truncatula cytokinin perception mutant cre1. Plant Cell 2015, 27, 2210–2226.
- Soltis, D.E.; Soltis, P.S.; Morgan, D.R.; Swensen, S.M.; Mullin, B.C.; Dowd, J.M.; Martin, P.G. Chloroplast gene sequence data suggest a single origin of the predisposition for symbiotic nitrogen fixation in angiosperms. Proc. Nat. Acad. Sci. USA 1995, 92, 2647–2651.
- Kistner, C.; Parniske, M. Evolution of signal transduction in intracellular symbiosis. Trends Plant Sci. 2002, 7, 511–518.
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323.
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555.
- Watkins, J.M.; Hechler, P.J.; Muday, G.K. Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiol. 2014, 164, 1707–1717.
- Watkins, J.M.; Chapman, J.M.; Muday, G.K. Abscisic acid-induced reactive oxygen species are modulated by flavonols to control stomata aperture. Plant Physiol. 2017, 175, 1807–1825.
- Watkins, J.M.; Hechler, P.J.; Muday, G.K. Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiol. 2014, 164, 1707–1717.
- Watkins, J.M.; Chapman, J.M.; Muday, G.K. Abscisic acid-induced reactive oxygen species are modulated by flavonols to control stomata aperture. Plant Physiol. 2017, 175, 1807–1825.
