Honeybees are the most prevalent insect pollinator species; they pollinate a wide range of crops. Colony collapse disorder (CCD), which is caused by a variety of biotic and abiotic factors, incurs high economic/ecological loss. Various ecological stressors are microbial infections, exposure to pesticides, loss of habitat, and improper beekeeping practices that are claimed to cause these declines. Honeybees have an innate immune system, which includes physical barriers and cellular and humeral responses to defend against pathogens and parasites. Exposure to various stressors may affect this system and the health of individual bees and colonies.
- honeybees
- immunity
- ecological stressors
- sustainable beekeeping
1. Introduction
2. Honeybee Immunity
3. Main Causes of Honeybee Colony Losses
3.1. Varroa Mite
3.2. Nosema spp.
3.3. Viral Pathogens
3.4. Pesticides
3.5. Malnutrition
3.6. Other Causes
4. Interaction between Different Stressors Affects the Bees Immunocompetence
4.1. Interaction between Pesticides and Pathogens
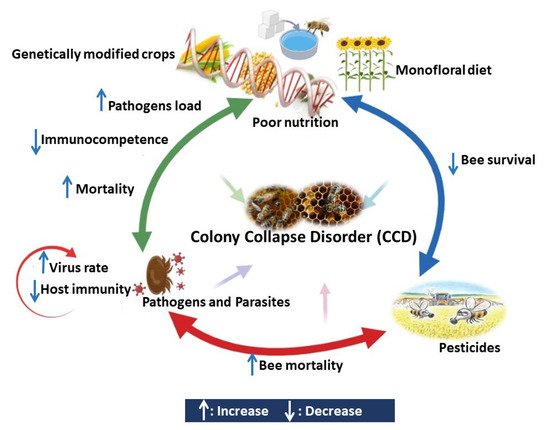
4.2. Interaction between Pesticides and Poor Nutrition
4.3. Interaction between Pathogens and Poor Nutrition
4.4. Interaction between Parasites and Pathogens
5. Strategies to Enhance Honeybee Immunity
5.1. Fortified Nutrients
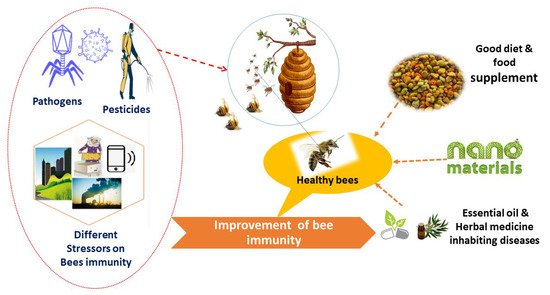
5.2. Natural Products as Alternative Sources
Essential oils such as thymol, linalool, and camphor, as well as cocktails of thymol, eucalyptol, menthol, and others, have been confirmed to be particularly efficient in suppressing Varroa mites. These types of essential oils were discovered to lower mortality rates among bees in diseased colonies [168]. Although natural products therapy has fewer side effects than chemical therapy, the efficacy of these substances varies depending on the climate and colony condition [169].
Recently, Chinese herbal medicine has demonstrated a unique antiviral effect for both human and animal life. Honeybees are at risk from SBV and Chinese sacbrood virus (CSBV). Infected larvae will not develop into pupae and will eventually die, and there is currently no effective cure for the virus [20]. Radix isatidis, a Chinese herbal remedy, was primarily utilized to treat human influenza viruses. It has recently been proved to effectively regulate CSBV by suppressing its replication, increasing immunological response, and extending the lifespan of CSBV infection larvae, thereby lowering death rates and preventing CCD [170]. DWV and Lake Sinai virus are two RNA viruses with positive strands that kill honeybees. Bees fed with polypore mushroom extracts exhibited a strong ability to diminish both virus larvae. Modified porphyrins, which are mostly produced by living organisms, can reduce spore burdens in bees and increase the survival likelihood of bees infected with RNA viruses [171].
5.3. Nanomaterials as Novel Alternative Approaches
5.4. Organizations and Initiatives Directed to Saving the Bees
6. Conclusions
References
- Stein, K.; Coulibaly, D.; Stenchly, K.; Goetze, D.; Porembski, S.; Lindner, A.; Konaté, S.; Linsenmair, E.K. Bee pollination increases yield quantity and quality of cash crops in Burkina Faso, West Africa. Rep. 2017, 7, 17691–17700. https://doi.org/10.1038/s41598-017-17970-2.
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; Alajmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of bee pollination and its economic value for crop production. Insects 2021, 12, 688–703. https://doi.org/10.3390/insects12080688.
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. R. Soc. B Biol. Sci. 2007, 274, 303–313. https://doi.org/10.1098/rspb.2006.3721.
- Hristov, P.; Neov, B.; Shumkova, R.; Palova, N. Significance of apoidea as main pollinators. Ecological and economic impact and implications for human nutrition. Diversity 2020, 12, 280–294. https://doi.org/10.3390/d12070280.
- Dicks, L.V.; Breeze, T.D.; Ngo, H.T.; Senapathi, D.; An, J.; Aizen, M.A.; Basu, P.; Buchori, D.; Galetto, L.; Garibaldi, L.A.; et al. A global-scale expert assessment of drivers and risks associated with pollinator decline. Ecol. Evol. 2021, 5, 1453–1461. https://doi.org/10.1038/s41559-021-01534-9.
- Goodrich, B.K. Do more bees imply higher fees? Honey bee colony strength as a determinant of almond pollination fees. Food Policy 2019, 83, 150–160. https://doi.org/10.1016/j.foodpol.2018.12.008.
- VanEngelsdorp, D.; Traynor, K.S.; Andree, M.; Lichtenberg, E.M.; Chen, Y.; Saegerman, C.; Cox-Foster, D.L. Colony collapse disorder (CCD) and bee age impact honey bee pathophysiology. PLoS ONE 2017, 12, e0179535. https://doi.org/10.1371/journal.pone.0179535.
- vanEngelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. Invertebr. Pathol. 2010, 103, S80–S95. https://doi.org/10.1016/j.jip.2009.06.011.
- Al Naggar, Y.; Baer, B. Consequences of a short time exposure to a sublethal dose of Flupyradifurone (Sivanto) pesticide early in life on survival and immunity in the honeybee (Apis mellifera). Rep. 2019, 9, 19753–19762. https://doi.org/10.1038/s41598-019-56224-1.
- Al Naggar, Y.; Paxton, R.J. Mode of transmission determines the virulence of black queen cell virus in adult honey bees, posing a future threat to bees and apiculture. Viruses 2020, 12, 535–546.
- Al Naggar, Y.; Paxton, R.J. The novel insecticides flupyradifurone and sulfoxaflor do not act synergistically with viral pathogens in reducing honey bee (Apis mellifera) survival but sulfoxaflor modulates host immunocompetence. Biotechnol. 2021, 14, 227–240. https://doi.org/10.1111/1751-7915.13673.
- Jacques, A.; Laurent, M.; Consortium, E.; Ribière-Chabert, M.; Saussac, M.; Bougeard, S.; Budge, G.E.; Hendrikx, P.; Chauzat, M.-P. A pan-European epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. PLoS ONE 2017, 12, e0172591.
- Mullapudi, E.; Přidal, A.; Pálková, L.; de Miranda, J.R.; Plevka, P. Virion structure of Israeli acute bee paralysis virus. Virol. 2016, 90, 8150–8159. https://doi.org/10.1128/jvi.00854-16.
- van Engelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony collapse disorder: A descriptive study. PLoS ONE 2009, 4, e6481. https://doi.org/10.1371/journal.pone.0006481.
- Nazzi, F.; Annoscia, D.; Caprio, E.; Di Prisco, G.; Pennacchio, F. Honeybee immunity and colony losses. Entomologia 2014, 2, 80-87. https://doi.org/10.4081/entomologia.2014.203.
- Antúnez, K.; Martín-Hernández, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Microbiol. 2009, 11, 2284–2290. https://doi.org/10.1111/j.1462-2920.2009.01953.x.
- Fallon, J.P.; Troy, N.; Kavanagh, K. Pre-exposure of Galleria mellonella larvae to different doses of Aspergillus fumigatus conidia causes differential activation of cellular and humoral immune responses. Virulence 2011, 2, 413–421.
- DeGrandi-Hoffman, G.; Chen, Y. Nutrition, immunity and viral infections in honey bees. Opin. Insect Sci. 2015, 10, 170–176.
- Karlikow, M.; Goic, B.; Saleh, M.-C. RNAi and antiviral defense in Drosophila: Setting up a systemic immune response. Comp. Immunol. 2014, 42, 85–92.
- Brutscher, L.M.; Flenniken, M.L. RNAi and antiviral defense in the honey bee. Immunol. Res. 2015, 2015, 941897. https://doi.org/10.1155/2015/941897.
- Vung, N.N.; Choi, Y.S.; Kim, I. High resistance to Sacbrood virus disease in Apis cerana (Hymenoptera: Apidae) colonies selected for superior brood viability and hygienic behavior. Apidologie 2020, 51, 61–74.
- Goblirsch, M.; Warner, J.F.; Sommerfeldt, B.A.; Spivak, M. Social fever or general immune response? Revisiting an example of social immunity in honey bees. Insects 2020, 11, 528–539. https://doi.org/10.3390/insects11080528.
- Cini, A.; Bordoni, A.; Cappa, F.; Petrocelli, I.; Pitzalis, M.; Iovinella, I.; Dani, F.R.; Turillazzi, S.; Cervo, R. Increased immunocompetence and network centrality of allogroomer workers suggest a link between individual and social immunity in honeybees. Rep. 2020, 10, 8928–8939.
- Simone, M.; Evans, J.D.; Spivak, M. Resin collection and social immunity in honey bees. Int. J. Org. Evol. 2009, 63, 3016–3022.
- Borba, R.S.; Spivak, M. Propolis envelope in Apis mellifera colonies supports honey bees against the pathogen, Paenibacillus larvae. Rep. 2017, 7, 11429–11440.
- Bucekova, M.; Valachova, I.; Kohutova, L.; Prochazka, E.; Klaudiny, J.; Majtan, J. Honeybee glucose oxidase—its expression in honeybee workers and comparative analyses of its content and H2O2-mediated antibacterial activity in natural honeys. Naturwissenschaften 2014, 101, 661–670.
- Brudzynski, K. Effect of hydrogen peroxide on antibacterial activities of Canadian honeys. J. Microbiol. 2006, 52, 1228–1237. https://doi.org/10.1139/W06-086.
- Alkhatib, A. Antiviral functional foods and exercise lifestyle prevention of coronavirus. Nutrients 2020, 12, 2633–2649. https://doi.org/10.3390/nu12092633.
- Roger, N.; Michez, D.; Wattiez, R.; Sheridan, C.; Vanderplanck, M. Diet effects on bumblebee health. Insect Physiol. 2017, 96, 128–133.
- Aronstein, K.A.; Saldivar, E.; Vega, R.; Westmiller, S.; Douglas, A.E. How Varroa parasitism affects the immunological and nutritional status of the honey bee, Apis mellifera. Insects 2012, 3, 601–615.
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016.
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. https://doi.org/10.1126/science.1255957.
- Dolezal, A.G.; Toth, A.L. Feedbacks between nutrition and disease in honey bee health. Opin. Insect Sci. 2018, 26, 114–119. https://doi.org/10.1016/j.cois.2018.02.006.
- Gebremedhn, H.; Amssalu, B.; De Smet, L.; De Graaf, D.C. Factors restraining the population growth of Varroa destructor in Ethiopian honey bees (Apis mellifera simensis). PLoS ONE 2019, 14, e0223236. https://doi.org/10.1371/journal.pone.0223236.
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Natl. Acad. Sci. USA 2019, 116, 1792–1801. https://doi.org/10.1073/pnas.1818371116.
- De Figueiró Santos, J.; Coelho, F.C.; Bliman, P.A. Behavioral modulation of infestation by varroa destructor in bee colonies. Implications for colony stability. PLoS ONE 2016, 11, e0160465. https://doi.org/10.1371/journal.pone.0160465.
- González-Cabrera, J.; Bumann, H.; Rodríguez-Vargas, S.; Kennedy, P.J.; Krieger, K.; Altreuther, G.; Hertel, A.; Hertlein, G.; Nauen, R.; Williamson, M.S. A single mutation is driving resistance to pyrethroids in European populations of the parasitic mite, Varroa destructor. Pest Sci. 2018, 91, 1137–1144. https://doi.org/10.1007/s10340-018-0968-y.
- Morawetz, L.; Köglberger, H.; Griesbacher, A.; Derakhshifar, I.; Crailsheim, K.; Brodschneider, R.; Moosbeckhofer, R. Health status of honey bee colonies (Apis mellifera) and disease-related risk factors for colony losses in Austria. PLoS ONE 2019, 14, e0219293. https://doi.org/10.1371/journal.pone.0219293.
- Richards, E.H.; Jones, B.; Bowman, A. Salivary secretions from the honeybee mite, Varroa destructor: Effects on insect haemocytes and preliminary biochemical characterization. Parasitology 2011, 138, 602–608.
- Koleoglu, G.; Goodwin, P.H.; Reyes-Quintana, M.; Guzman-Novoa, E. Effect of Varroa destructor, counding and Varroa homogenate on gene expression in brood and adult honey bees. PLoS ONE 2017, 12, e0169669. https://doi.org/10.1371/journal.pone.0169669.
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Della Vedova, G.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog. 2012, 8, e1002735. https://doi.org/10.1371/journal.ppat.1002735.
- Kielmanowicz, M.G.; Inberg, A.; Lerner, I.M.; Golani, Y.; Brown, N.; Turner, C.L.; Hayes, G.J.R.; Ballam, J.M. Prospective large-scale field study generates predictive model identifying major contributors to colony losses. PLoS Pathog. 2015, 11, e1004816. https://doi.org/10.1371/journal.ppat.1004816.
- Di Prisco, G.; Pennacchio, F.; Caprio, E.; Boncristiani, H.F.; Evans, J.D.; Chen, Y. Varroa destructor is an effective vector of Israeli acute paralysis virus in the honeybee, Apis mellifera. Gen. Virol. 2011, 92, 151–155. https://doi.org/10.1099/vir.0.023853-0.
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive markers of honey bee colony collapse. PLoS ONE 2012, 7, e32151. https://doi.org/10.1371/journal.pone.0032151.
- Gisder, S.; Schüler, V.; Horchler, L.L.; Groth, D.; Genersch, E. Long-term temporal trends of Nosema infection prevalence in Northeast Germany: Continuous spread of Nosema ceranae, an emerging pathogen of honey bees (Apis mellifera), but no general replacement of Nosema apis. Front. Cell. Infect. Microbiol. 2017, 7, 301–314. https://doi.org/10.3389/fcimb.2017.00301.
- Higes, M.; Martín-Hernández, R.; Botías, C.; Bailón, E.G.; González-Porto, A.V.; Barrios, L.; Del Nozal, M.J.; Bernal, J.L.; Jiménez, J.J.; Palencia, P.G.; et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Microbiol. 2008, 10, 2659–2669. https://doi.org/10.1111/j.1462-2920.2008.01687.x.
- Dosselli, R.; Grassl, J.; Carson, A.; Simmons, L.W.; Baer, B. Flight behaviour of honey bee (Apis mellifera) workers is altered by initial infections of the fungal parasite Nosema apis. Rep. 2016, 6, 36649–36659. https://doi.org/10.1038/srep36649.
- Dussaubat, C.; Maisonnasse, A.; Crauser, D.; Beslay, D.; Costagliola, G.; Soubeyrand, S.; Kretzchmar, A.; Le Conte, Y. Flight behavior and pheromone changes associated to Nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. Invertebr. Pathol. 2013, 113, 42–51. https://doi.org/10.1016/j.jip.2013.01.002.
- Li, W.; Evans, J.D.; Li, J.; Su, S.; Hamilton, M.; Chen, Y. Spore load and immune response of honey bees naturally infected by Nosema ceranae. Res. 2017, 116, 3265–3274.
- Grozinger, C.M.; Flenniken, M.L. Bee viruses: Ecology, pathogenicity, and impacts. Rev. Entomol. 2019, 64, 205–226. https://doi.org/10.1146/annurev-ento-011118-111942.
- Škubnik, K.; Nováček, J.; Füzik, T.; Přidal, A.; Paxton, R.J.; Plevka, P. Structure of deformed wing virus, a major honey bee pathogen. Natl. Acad. Sci. USA 2017, 114, 3210–3215. https://doi.org/10.1073/pnas.1615695114.
- Quintana, S.; Brasesco, C.; Negri, P.; Marin, M.; Pagnuco, I.; Szawarski, N.; Reynaldi, F.; Larsen, A.; Eguaras, M.; Maggi, M. Up-regulated pathways in response to Deformed Wing Virus infection in Apis mellifera (Hymenoptera: Apidae). Soc. Entomol. Argent. 2019, 78, 1–11.
- Barroso-Arévalo, S.; Vicente-Rubiano, M.; Puerta, F.; Molero, F.; Sánchez-Vizcaíno, J.M. Immune related genes as markers for monitoring health status of honey bee colonies. BMC Vet. Res. 2019, 15, 72–86. https://doi.org/10.1186/s12917-019-1823-y.
- Di, G.; Annoscia, D.; Margiotta, M.; Ferrara, R.; Varricchio, P.; Zanni, V. A mutualistic symbiosis between a parasitic mite and a pathogenic virus undermines honey bee immunity and health. Natl. Acad. Sci. USA 2016, 113, 3203–3208. https://doi.org/10.1073/pnas.1523515113.
- Ryabov, E.V.; Fannon, J.M.; Moore, J.D.; Wood, G.R.; Evans, D.J. The iflaviruses sacbrood virus and deformed wing virus evoke different transcriptional responses in the honeybee which may facilitate their horizontal or vertical transmission. PeerJ 2016, 4, e1591. https://doi.org/10.7717/peerj.1591.
- Smart, M.; Pettis, J.; Rice, N.; Browning, Z.; Spivak, M. Linking measures of colony and individual honey bee health to survival among apiaries exposed to varying agricultural land use. PLoS ONE 2016, 11, e0152685.
- Abbo, P.M.; Kawasaki, J.K.; Hamilton, M.; Cook, S.C.; DeGrandi-Hoffman, G.; Li, W.F.; Liu, J.; Chen, Y.P. Effects of Imidacloprid and Varroa destructor on survival and health of European honey bees, Apis mellifera. Insect Sci. 2017, 24, 467–477. https://doi.org/10.1111/1744-7917.12335.
- Li, J.; Huang, S.; Heerman, M.; Rodr, C.; Banmeke, O.; Brister, J.R.; Hatcher, E.L.; Cao, L.; Hamilton, M.; Chen, Y. The phylogeny and pathogenesis of sacbrood virus (SBV) infection in european honey bees, Apis mellifera. Viruses 2019, 11, 61–77. https://doi.org/10.3390/v11010061.
- Shan, L.; Liuhao, W.; Jun, G.; Yujie, T.; Yanping, C.; Jie, W.; Jilian, L. Chinese Sacbrood virus infection in Asian honey bees (Apis cerana cerana) and host immune responses to the virus infection. Invertebr. Pathol. 2017, 150, 63–69.
- Cox-Foster, D.L.; Conlan, S.; Holmes, E.C.; Palacios, G.; Evans, J.D.; Moran, N.A.; Quan, P.L.; Briese, T.; Hornig, M.; Geiser, D.M.; et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 2007, 318, 283–287. https://doi.org/10.1126/science.1146498.
- Johnson, R.M.; Evans, J.D.; Robinson, G.E.; Berenbaum, M.R. Changes in transcript abundance relating to colony collapse disorder in honey bees (Apis mellifera). Natl. Acad. Sci. USA 2009, 106, 14790–14795. https://doi.org/10.1073/pnas.0906970106.
- Hou, C.; Rivkin, H.; Slabezki, Y.; Chejanovsky, N. Dynamics of the presence of israeli acute paralysis virus in honey bee colonies with colony collapse disorder. Viruses 2014, 6, 2012–2027. https://doi.org/10.3390/v6052012.
- Cornman, R.S.; Tarpy, D.R.; Chen, Y.; Jeffreys, L.; Lopez, D.; Pettis, J.S.; VanEngelsdorp, D.; Evans, J.D. Pathogen webs in collapsing honey bee colonies. PLoS ONE 2012, 7, e43562. https://doi.org/10.1371/journal.pone.0043562.
- Meeus, I.; de Miranda, J.R.; de Graaf, D.C.; Wäckers, F.; Smagghe, G. Effect of oral infection with Kashmir bee virus and Israeli acute paralysis virus on bumblebee (Bombus terrestris) reproductive success. Invertebr. Pathol. 2014, 121, 64–69.
- Alvarez, L.J.; Reynaldi, F.J.; Ramello, P.J.; Garcia, M.L.G.; Sguazza, G.H.; Abrahamovich, A.H.; Lucia, M. Detection of honey bee viruses in Argentinian stingless bees (Hymenoptera: Apidae). Insectes Soc. 2018, 65, 191–197. https://doi.org/10.1007/s00040-017-0587-2.
- Dalmon, A.; Gayral, P.; Decante, D.; Klopp, C.; Bigot, D.; Thomasson, M.; AHerniou, E.; Alaux, C.; Conte, Y. Le Viruses in the invasive hornet Vespa velutina. Viruses 2019, 11, 1041. https://doi.org/10.3390/v11111041.
- Payne, A.N.; Shepherd, T.F.; Rangel, J. The detection of honey bee (Apis mellifera)-associated viruses in ants. Rep. 2020, 10, 2923–2930.
- Chen, Y.P.; Pettis, J.S.; Corona, M.; Chen, W.P.; Li, C.J.; Spivak, M.; Visscher, P.K.; DeGrandi-Hoffman, G.; Boncristiani, H.; Zhao, Y. Israeli acute paralysis virus: Epidemiology, pathogenesis and implications for honey bee health. PLoS Pathog 2014, 10, e1004261.
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera. Natl. Acad. Sci. USA 2013, 110, 8842–8846. https://doi.org/10.1073/pnas.1303884110.
- Cresswell, J.E.; Thompson, H.M. Comment on “a common pesticide decreases foraging success and survival in honey bees.” Science 2012, 337, 1453. https://doi.org/10.1126/science.1224618.
- Tesovnik, T.; Cizelj, I.; Zorc, M.; Čitar, M.; Božič, J.; Glavan, G.; Narat, M. Immune related gene expression in worker honey bee (Apis mellifera carnica) pupae exposed to neonicotinoid thiamethoxam and Varroa mites (Varroa destructor). PLoS ONE 2017, 12, e0187079. https://doi.org/10.1371/journal.pone.0187079.
- Tesovnik, T.; Zorc, M.; Gregorc, A.; Rinehart, T.; Adamczyk, J.; Narat, M. Immune gene expression in developing honey bees (Apis mellifera) simultaneously exposed to imidacloprid and Varroa destructor in laboratory conditions. J. Apic. Res. 2019, 58, 730–739. https://doi.org/10.1080/00218839.2019.1634463.
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The neonicotinoids thiacloprid , imidacloprid , and clothianidin affect the immunocompetence of honey bees (Apis mellifera). J. Insect Physiol. 2016, 86, 40–47. https://doi.org/10.1016/j.jinsphys.2016.01.001.
- Osterman, J.; Wintermantel, D.; Locke, B.; Jonsson, O.; Semberg, E.; Onorati, P.; Forsgren, E.; Rosenkranz, P.; Rahbek-Pedersen, T.; Bommarco, R. Clothianidin seed-treatment has no detectable negative impact on honeybee colonies and their pathogens. Commun. 2019, 10, 692–704.
- Siviter, H.; Bailes, E.J.; Martin, C.D.; Oliver, T.R.; Koricheva, J.; Leadbeater, E.; Brown, M.J.F. Agrochemicals interact synergistically to increase bee mortality. Nature 2021, 596, 389–392.
- Alaux, C.; Kemper, N.; Kretzschmar, A.; Le Conte, Y. Brain, physiological and behavioral modulation induced by immune stimulation in honeybees (Apis mellifera): A potential mediator of social immunity? Brain Behav. Immun. 2012, 26, 1057–1060.
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294.
- Cotter, S.C.; Simpson, S.J.; Raubenheimer, D.; Wilson, K. Macronutrient balance mediates trade‐offs between immune function and life history traits. Ecol. 2011, 25, 186–198.
- Frizzera, D.; Del Fabbro, S.; Ortis, G.; Zanni, V.; Bortolomeazzi, R.; Nazzi, F.; Annoscia, D. Possible side effects of sugar supplementary nutrition on honey bee health. Apidologie 2020, 51, 594–608.
- Desneux, N.; Bernal, J.S. Genetically modified crops deserve greater ecotoxicological scrutiny. Ecotoxicology 2010, 19, 1642–1644.
- O’Callaghan, M.; Glare, T.R.; Burgess, E.P.J.; Malone, L.A. Effects of plants genetically modified for insect resistance on nontarget organisms. Rev. Entomol. 2005, 50, 271–292.
- Shelton, A.M.; Zhao, J.-Z.; Roush, R.T. Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Rev. Entomol. 2002, 47, 845–881.
- Qaim, M.; Kouser, S. Genetically modified crops and food security. PLoS ONE 2013, 8, e64879.
- Duan, J.J.; Marvier, M.; Huesing, J.; Dively, G.; Huang, Z.Y. A meta-analysis of effects of Bt crops on honey bees (Hymenoptera: Apidae). PLoS ONE 2008, 3, e1415.
- Devillers, J.; Pham-Delègue, M.-H. Using proteins to assess the poteinal impacts of genentically modifiedplants on honey bees. In Honey Bees: Estimating the Environmental Impact of Chemicals; CRC Press: USA 2002; pp. 290–304. ISBN 0203218655.
- Aldgini, H.M.M.; Al-Abbadi, A.A.; Abu-Nameh, E.S.M.; Alghazeer, R.O. Determination of metals as bio indicators in some selected bee pollen samples from Jordan. Saudi J. Biol. Sci. 2019, 26, 1418–1422.
- Nikolić, T.V.; Kojić, D.; Orčić, S.; Batinić, D.; Vukašinović, E.; Blagojević, D.P.; Purać, J. The impact of sublethal concentrations of Cu, Pb and Cd on honey bee redox status, superoxide dismutase and catalase in laboratory conditions. Chemosphere 2016, 164, 98–105.
- Polykretis, P.; Delfino, G.; Petrocelli, I.; Cervo, R.; Tanteri, G.; Montori, G.; Perito, B.; Branca, J.J.V.; Morucci, G.; Gulisano, M. Evidence of immunocompetence reduction induced by cadmium exposure in honey bees (Apis mellifera). Pollut. 2016, 218, 826–834. https://doi.org/10.1016/j.envpol.2016.08.006.
- Rothman, J.A.; Leger, L.; Kirkwood, J.S.; McFrederick, Q.S. Cadmium and selenate exposure affects the honey bee microbiome and metabolome, and bee-associated bacteria show potential for bioaccumulation. Environ. Microbiol. 2019, 85, e01411-19. https://doi.org/10.1128/AEM.01411-19.
- Sadowska, M.; Gogolewska, H.; Pawelec, N.; Sentkowska, A.; Krasnodębska-Ostręga, B. Comparison of the contents of selected elements and pesticides in honey bees with regard to their habitat. Sci. Pollut. Res. 2019, 26, 371–380. https://doi.org/10.1007/s11356-018-3612-8.
- Fisogni, A.; Hautekèete, N.; Piquot, Y.; Brun, M.; Vanappelghem, C.; Michez, D.; Massol, F. Urbanization drives an early spring for plants but not for pollinators. Oikos 2020, 129, 1681–1691. https://doi.org/10.1111/oik.07274.
- Appler, R.H.; Frank, S.D.; Tarpy, D.R. Within-colony variation in the immunocompetency of managed and feral honey bees (Apis mellifera) in different urban landscapes. Insects 2015, 6, 912–925. https://doi.org/10.3390/insects6040912.
- Dabour, K.; Al Naggar, Y.; Masry, S.; Naiem, E.; Giesy, J.P. Cellular alterations in midgut cells of honey bee workers (Apis millefera ) exposed to sublethal concentrations of CdO or PbO nanoparticles or their binary mixture. Sci. Total Environ. 2019, 651, 1356–1367. https://doi.org/10.1016/j.scitotenv.2018.09.311.
- AL Naggar, Y.; Dabour, K.; Masry, S.; Sadek, A.; Naiem, E.; Giesy, J.P. Sublethal effects of chronic exposure to CdO or PbO nanoparticles or their binary mixture on the honey bee (Apis millefera). Environ. Sci. Pollut. Res. 2020, 27, 19004–19015. https://doi.org/10.1007/s11356-018-3314-2.
- Sharma, V.P.; Kumar, N.R. Changes in honeybee behaviour and biology under the influence of cellphone radiations. Sci. 2010, 98, 1376–1378.
- Santhosh Kumar, S. Colony collapse disorder (CCD) in honey bees caused by EMF radiation. Bioinformation 2018, 14, 521–524. https://doi.org/10.6026/97320630014521.
- Lupi, D.; Tremolada, P.; Colombo, M.; Giacchini, R.; Benocci, R.; Parenti, P.; Parolini, M.; Zambon, G.; Vighi, M. Effects of pesticides and electromagnetic fields on honeybees: A field study using biomarkers. J. Environ. Res. 2020, 14, 107–122. https://doi.org/10.1007/s41742-019-00242-4.
- Odemer, R.; Odemer, F. Effects of radiofrequency electromagnetic radiation (RF-EMF) on honey bee queen development and mating success. Total Environ. 2019, 661, 553–562. https://doi.org/10.1016/j.scitotenv.2019.01.154.
- Clermont, A.; Eickermann, M.; Kraus, F.; Hoffmann, L.; Beyer, M. Correlations between land covers and honey bee colony losses in a country with industrialized and rural regions. Total Environ. 2015, 532, 1–13. https://doi.org/10.1016/j.scitotenv.2015.05.128.
- O’Neal, S.T.; Anderson, T.D.; Wu-Smart, J.Y. Interactions between pesticides and pathogen susceptibility in honey bees. Opin. Insect Sci. 2018, 26, 57–62. https://doi.org/10.1016/j.cois.2018.01.006.
- Pettis, J.S.; Vanengelsdorp, D.; Johnson, J.; Dively, G. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 2012, 99, 153–158. https://doi.org/10.1007/s00114-011-0881-1.
- Di, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Natl. Acad. Sci. USA 2013, 110, 18466–18471. https://doi.org/10.1073/pnas.1314923110.
- Dussaubat, C.; Maisonnasse, A.; Crauser, D.; Tchamitchian, S.; Bonnet, M.; Cousin, M.; Kretzschmar, A.; Brunet, J.-L.; Le Conte, Y. Combined neonicotinoid pesticide and parasite stress alter honeybee queens’ physiology and survival. Rep. 2016, 6, 31430–31436.
- Aufauvre, J.; Misme-Aucouturier, B.; Viguès, B.; Texier, C.; Delbac, F.; Blot, N. Transcriptome analyses of the honeybee response to Nosema ceranae and insecticides. PLoS ONE 2014, 9, e91686.
- Retschnig, G.; Neumann, P.; Williams, G.R. Thiacloprid-Nosema ceranae interactions in honey bees: Host survivorship but not parasite reproduction is dependent on pesticide dose. Invertebr. Pathol. 2014, 118, 18–19. https://doi.org/10.1016/j.jip.2014.02.008.
- Diao, Q.; Li, B.; Zhao, H.; Wu, Y.; Guo, R.; Dai, P.; Chen, D.; Wang, Q.; Hou, C. Enhancement of chronic bee paralysis virus levels in honeybees acute exposed to imidacloprid: A Chinese case study. Total Environ. 2018, 630, 487–494. https://doi.org/10.1016/j.scitotenv.2018.02.258.
- Tosi, S.; Nieh, J.C.; Sgolastra, F.; Cabbri, R.; Medrzycki, P. Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. R. Soc. B Biol. Sci. 2017, 284, 20171711–20171719. https://doi.org/10.1098/rspb.2017.1711.
- Sánchez-bayo, F.; Goulson, D.; Pennacchio, F.; Nazzi, F.; Goka, K.; Desneux, N. Are bee diseases linked to pesticides ?—A brief review. Int. 2016, 89–90, 7–11. https://doi.org/10.1016/j.envint.2016.01.009.
- Wu-Smart, J.; Spivak, M. Sub-lethal effects of dietary neonicotinoid insecticide exposure on honey bee queen fecundity and colony development. Rep. 2016, 6, 32108–32119.
- Tong, L.; Nieh, J.C.; Tosi, S. Combined nutritional stress and a new systemic pesticide (flupyradifurone, Sivanto®) reduce bee survival, food consumption, flight success, and thermoregulation. Chemosphere 2019, 237, 124408–12416.
- Foley, K.; Fazio, G.; Jensen, A.B.; Hughes, W.O.H. Nutritional limitation and resistance to opportunistic Aspergillus parasites in honey bee larvae. Invertebr. Pathol. 2012, 111, 68–73.
- Koch, H.; Brown, M.J.; Stevenson, P.C. The role of disease in bee foraging ecology. Opin. Insect Sci. 2017, 21, 60–67. https://doi.org/10.1016/j.cois.2017.05.008.
- Dolezal, A.G.; Carrillo-Tripp, J.; Judd, T.M.; Allen Miller, W.; Bonning, B.C.; Toth, A.L. Interacting stressors matter: Diet quality and virus infection in honeybee health. Soc. Open Sci. 2019, 6, 181803–181813.
- Zhao, Y.; Heerman, M.; Peng, W.; Evans, J.D.; Rose, R.; DeGrandi-Hoffman, G.; Simone-Finstrom, M.; Li, J.; Li, Z.; Cook, S.C. The dynamics of deformed wing virus concentration and host defensive gene expression after Varroa mite parasitism in honey bees, Apis mellifera. Insects 2019, 10, 16–34.
- Straub, L.; Williams, G.R.; Vidondo, B.; Khongphinitbunjong, K.; Retschnig, G.; Schneeberger, A.; Chantawannakul, P.; Dietemann, V.; Neumann, P. Neonicotinoids and ectoparasitic mites synergistically impact honeybees. Rep. 2019, 9, 8159–8168. https://doi.org/10.1038/s41598-019-44207-1.
- Annoscia, D.; Brown, S.P.; Di Prisco, G.; De Paoli, E.; Del Fabbro, S.; Frizzera, D.; Zanni, V.; Galbraith, D.A.; Caprio, E.; Grozinger, C.M. Haemolymph removal by Varroa mite destabilizes the dynamical interaction between immune effectors and virus in bees, as predicted by Volterra’s model. R. Soc. B 2019, 286, 20190331–20190339.
- van Dooremalen, C.; Stam, E.; Gerritsen, L.; Cornelissen, B.; van der Steen, J.; van Langevelde, F.; Blacquière, T. Interactive effect of reduced pollen availability and Varroa destructor infestation limits growth and protein content of young honey bees. Insect Physiol. 2013, 59, 487–493. https://doi.org/10.1016/j.jinsphys.2013.02.006.
- Aufauvre, J.; Biron, D.G.; Vidau, C.; Fontbonne, R.; Roudel, M.; Diogon, M.; Viguès, B.; Belzunces, L.P.; Delbac, F.; Blot, N. Parasite-insecticide interactions: A case study of Nosema ceranae and fipronil synergy on honeybee. Rep. 2012, 2, 326–332. https://doi.org/10.1038/srep00326.
- Zheng, H.-Q.; Gong, H.-R.; Huang, S.-K.; Sohr, A.; Hu, F.-L.; Chen, Y.P. Evidence of the synergistic interaction of honey bee pathogens Nosema ceranae and deformed wing virus. Microbiol. 2015, 177, 1–6.
- Alaux, C.; Brunet, J.; Dussaubat, C.; Mondet, F.; Tchamitchan, S.; Cousin, M.; Brillard, J.; Baldy, A.; Belzunces, L.P.; Le Conte, Y. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Microbiol. 2010, 12, 774–782.
- Tesovnik, T.; Zorc, M.; Ristanić, M.; Glavinić, U.; Stevanović, J.; Narat, M.; Stanimirović, Z. Exposure of honey bee larvae to thiamethoxam and its interaction with Nosema ceranae infection in adult honey bees. Pollut. 2020, 256, 113443.
- Coulon, M.; Schurr, F.; Martel, A.-C.; Cougoule, N.; Bégaud, A.; Mangoni, P.; Di Prisco, G.; Dalmon, A.; Alaux, C.; Ribière-Chabert, M. Influence of chronic exposure to thiamethoxam and chronic bee paralysis virus on winter honey bees. PLoS ONE 2019, 14, e0220703.
- Coulon, M.; Schurr, F.; Martel, A.-C.; Cougoule, N.; Bégaud, A.; Mangoni, P.; Dalmon, A.; Alaux, C.; Le Conte, Y.; Thiéry, R. Metabolisation of thiamethoxam (a neonicotinoid pesticide) and interaction with the chronic bee paralysis virus in honeybees. Biochem. Physiol. 2018, 144, 10–18.
- De Miranda, J.R.; Doublet, V.; Labarussias, M.; Miranda, J.R. De; Moritz, R.F.A.; Paxton, R.J. Bees under stress: Sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Microbiol. 2015, 17, 969–983. https://doi.org/10.1111/1462-2920.12426.
- Odemer, R.; Nilles, L.; Linder, N.; Rosenkranz, P. Sublethal effects of clothianidin and Nosema on the longevity and foraging activity of free flying honey bees. Ecotoxicology 2018, 27, 527–538.
- Vidau, C.; Diogon, M.; Aufauvre, J.; Fontbonne, R.; Viguès, B.; Brunet, J.-L.; Texier, C.; Biron, D.G.; Blot, N.; El Alaoui, H. Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PLoS ONE 2011, 6, e21550.
- Morfin, N.; Goodwin, P.H.; Guzman-Novoa, E. Interaction of varroa destructor and sublethal clothianidin doses during the larval stage on subsequent adult honey bee (Apis mellifera) health, cellular immunity, deformed wing virus levels and differential gene expression. Microorganisms 2020, 8, 858–873. https://doi.org/10.3390/microorganisms8060858.
- Reyes-quintana, M.; Espinosa-montaño, L.G.; Prieto-merlos, D.; Koleoglu, G.; Petukhova, T.; Correa-benítez, A.; Guzman-novoa, E. Impact of Varroa destructor and deformed wing virus on emergence , cellular immunity , wing integrity and survivorship of Africanized honey bees in Mexico. Invertebr. Pathol. 2019, 164, 43–48. https://doi.org/10.1016/j.jip.2019.04.009.
- Blanken, L.J.; van Langevelde, F.; van Dooremalen, C. Interaction between Varroa destructor and imidacloprid reduces flight capacity of honeybees. R. Soc. B Biol. Sci. 2015, 282, 20151738.
- Francis, R.M.; Nielsen, S.L.; Kryger, P. Varroa-virus interaction in collapsing honey bee colonies. PLoS ONE 2013, 8, e57540..
- Almasri, H.; Tavares, D.A.; Diogon, M.; Pioz, M.; Alamil, M.; Sené, D.; Tchamitchian, S.; Cousin, M.; Brunet, J.-L.; Belzunces, L.P. Physiological effects of the interaction between Nosema ceranae and sequential and overlapping exposure to glyphosate and difenoconazole in the honey bee Apis mellifera. Environ. Saf. 2021, 217, 112258–112268.
- Grassl, J.; Holt, S.; Cremen, N.; Peso, M.; Hahne, D.; Baer, B. Synergistic effects of pathogen and pesticide exposure on honey bee (Apis mellifera) survival and immunity. Invertebr. Pathol. 2018, 159, 78–86. https://doi.org/10.1016/j.jip.2018.10.005.
- van Dooremalen, C.; Cornelissen, B.; Poleij-Hok-Ahin, C.; Blacquière, T. Single and interactive effects of Varroa destructor, Nosema, and imidacloprid on honey bee colonies (Apis mellifera). Ecosphere 2018, 9, e02378. https://doi.org/10.1002/ecs2.2378.
- Porrini, M.P.; Garrido, P.M.; Umpiérrez, M.L.; Porrini, L.P.; Cuniolo, A.; Davyt, B.; González, A.; Eguaras, M.J.; Rossini, C. Effects of synthetic acaricides and Nosema ceranae (microsporidia: Nosematidae) on molecules associated with chemical communication and recognition in honey bee. Sci. 2020, 7, 199–216.
- Bird, G.; Wilson, A.E.; Williams, G.R.; Hardy, N.B. Parasites and pesticides act antagonistically on honey bee health. Appl. Ecol. 2021, 58, 997–1005. https://doi.org/10.1111/1365-2664.13811.
- Wintermantel, D.; Locke, B.; Andersson, G.K.S.; Semberg, E.; Forsgren, E.; Osterman, J.; Pedersen, T.R.; Bommarco, R.; Smith, H.G.; Rundlöf, M. Field-level clothianidin exposure affects bumblebees but generally not their pathogens. Commun. 2018, 9, 5446–5455.
- Cutler, G.C.; Scott-Dupree, C.D.; Sultan, M.; McFarlane, A.D.; Brewer, L. A large-scale field study examining effects of exposure to clothianidin seed-treated canola on honey bee colony health, development, and overwintering success. PeerJ 2014, 2, e652.
- Rolke, D.; Fuchs, S.; Grünewald, B.; Gao, Z.; Blenau, W. Large-scale monitoring of effects of clothianidin-dressed oilseed rape seeds on pollinating insects in Northern Germany: Effects on honey bees (Apis mellifera). Ecotoxicology 2016, 25, 1648–1665.
- Faita, M.R.; Cardozo, M.M.; Amandio, D.T.T.; Orth, A.I.; Nodari, R.O. Glyphosate-based herbicides and Nosema sp. microsporidia reduce honey bee (Apis mellifera) survivability under laboratory conditions. J. Apic. Res. 2020, 59, 332–342. https://doi.org/10.1080/00218839.2020.1736782.
- Gregorc, A.; Silva-Zacarin, E.C.M.; Carvalho, S.M.; Kramberger, D.; Teixeira, E.W.; Malaspina, O. Effects of Nosema ceranae and thiametoxam in Apis mellifera: A comparative study in Africanized and Carniolan honey bees. Chemosphere 2016, 147, 328–336. https://doi.org/10.1016/j.chemosphere.2015.12.030.
- Coulon, M.; Dalmon, A.; Di Prisco, G.; Prado, A.; Arban, F.; Dubois, E.; Ribière-Chabert, M.; Alaux, C.; Thiéry, R.; Le Conte, Y. Interactions between thiamethoxam and deformed wing virus can drastically impair flight behavior of honey bees. Microbiol. 2020, 11, 766–777.
- Morfin, N.; Goodwin, P.H.; Guzman-Novoa, E. Interaction of field realistic doses of clothianidin and Varroa destructor parasitism on adult honey bee (Apis mellifera) health and neural gene expression, and antagonistic effects on differentially expressed genes. PLoS ONE 2020, 15, e0229030.
- Paris, L.; Peghaire, E.; Mone, A.; Diogon, M.; Debroas, D.; Delbac, F.; El Alaoui, H. Honeybee gut microbiota dysbiosis in pesticide/parasite co-exposures is mainly induced by Nosema ceranae. Invertebr. Pathol. 2020, 172, 107348–1073456.
- López, J.H.; Krainer, S.; Engert, A.; Schuehly, W.; Riessberger-Gallé, U.; Crailsheim, K. Sublethal pesticide doses negatively affect survival and the cellular responses in American foulbrood-infected honeybee larvae. Rep. 2017, 7, 40853–40865.
- Dance, C.; Botías, C.; Goulson, D. The combined effects of a monotonous diet and exposure to thiamethoxam on the performance of bumblebee micro-colonies. Environ. Saf. 2017, 139, 194–201.
- Renzi, M.T.; Rodríguez-Gasol, N.; Medrzycki, P.; Porrini, C.; Martini, A.; Burgio, G.; Maini, S.; Sgolastra, F. Combined effect of pollen quality and thiamethoxam on hypopharyngeal gland development and protein content in Apis mellifera. Apidologie 2016, 47, 779–788.
- Sperandio, G.; Simonetto, A.; Carnesecchi, E.; Costa, C.; Hatjina, F.; Tosi, S.; Gilioli, G. Beekeeping and honey bee colony health: A review and conceptualization of beekeeping management practices implemented in Europe. Total Environ. 2019, 696, 133795–133806.
- Zhang, G.; St. Clair, A.L.; Dolezal, A.; Toth, A.L.; O’Neal, M. Honey bee (hymenoptera: Apidea) pollen forage in a highly cultivated agroecosystem: Limited diet diversity and its relationship to virus resistance. Econ. Entomol. 2020, 113, 1062–1072. https://doi.org/10.1093/jee/toaa055.
- Jack, C.J.; Uppala, S.S.; Lucas, H.M.; Sagili, R.R. Effects of pollen dilution on infection of Nosema ceranae in honey bees. Insect Physiol. 2016, 87, 12–19.
- Dolezal, A.G.; Clair, A.L.S.; Zhang, G.; Toth, A.L.; O’Neal, M.E. Native habitat mitigates feast–famine conditions faced by honey bees in an agricultural landscape. Natl. Acad. Sci. USA 2019, 116, 25147–25155. https://doi.org/10.1073/pnas.1912801116.
- Castelli, L.; Branchiccela, B.; Garrido, M.; Invernizzi, C.; Porrini, M.; Romero, H.; Santos, E.; Zunino, P.; Antúnez, K. Impact of nutritional stress on honeybee gut microbiota, immunity, and Nosema ceranae Microb. Ecol. 2020, 80, 908–919.
- de Jong, E.W.; DeGrandi-Hoffman, G.; Chen, Y.; Graham, H.; Ziolkowski, N. Effects of diets containing different concentrations of pollen and pollen substitutes on physiology, Nosema burden, and virus titers in the honey bee (Apis mellifera ). Apidologie 2019, 50, 845–858.
- Ricigliano, V.A.; Dong, C.; Richardson, L.T.; Donnarumma, F.; Williams, S.T.; Solouki, T.; Murray, K.K. Honey bee proteome responses to plant and cyanobacteria (blue-green algae) diets. ACS Food Sci. Technol. 2020, 1, 17–26.
- Jehlík, T.; Kodrík, D.; Krištůfek, V.; Koubová, J.; Sábová, M.; Danihlík, J.; Tomčala, A.; Čapková Frydrychová, R. Effects of Chlorella sp. on biological characteristics of the honey bee Apis mellifera. Apidologie 2019, 50, 564–577. https://doi.org/10.1007/s13592-019-00670-3.
- Charistos, L.; Parashos, N.; Hatjina, F. Long term effects of a food supplement HiveAliveTM on honey bee colony strength and Nosema ceranae spore counts. Apic. Res. 2015, 54, 420–426. https://doi.org/10.1080/00218839.2016.1189231.
- Alberoni, D.; Baffoni, L.; Gaggìa, F.; Ryan, P.M.; Murphy, K.; Ross, P.R.; Stanton, C.; Di Gioia, D. Impact of beneficial bacteria supplementation on the gut microbiota, colony development and productivity of Apis mellifera Benef. Microbes 2018, 9, 269–278. https://doi.org/10.3920/BM2017.0061.
- Mitton, G.A.; Szawarski, N.; Mitton, F.M.; Iglesias, A.; Eguaras, M.J.; Ruffinengo, S.R.; Maggi, M.D. Impacts of dietary supplementation with p-coumaric acid and indole-3-acetic acid on survival and biochemical response of honey bees treated with tau-fluvalinate. Environ. Saf. 2020, 189, 109917–109924. https://doi.org/10.1016/j.ecoenv.2019.109917.
- Szawarski, N.; Saez, A.; Domínguez, E.; Dickson, R.; De Matteis, Á.; Eciolaza, C.; Justel, M.; Aliano, A.; Solar, P.; Bergara, I. Effect of abscisic acid (ABA) combined with two different beekeeping nutritional strategies to confront overwintering: Studies on honey bees’ population dynamics and nosemosis. Insects 2019, 10, 239–342.
- Lamontagne-Drolet, M.; Samson-Robert, O.; Giovenazzo, P.; Fournier, V. The impacts of two protein supplements on commercial honey bee (Apis mellifera) colonies. J. Apic. Res. 2019, 58, 800–813. https://doi.org/10.1080/00218839.2019.1644938.
- Cilia, G.; Fratini, F.; Tafi, E.; Turchi, B.; Mancini, S.; Sagona, S.; Nanetti, A.; Cerri, D.; Felicioli, A. Microbial profile of the ventriculum of honey bee (Apis mellifera ligustica spinola, 1806) fed with veterinary drugs, dietary supplements and non-protein amino acids. Sci. 2020, 7, 76. https://doi.org/10.3390/VETSCI7020076.
- Jovanovic, N.M.; Glavinic, U.; Delic, B.; Vejnovic, B.; Aleksic, N.; Mladjan, V.; Stanimirovic, Z. Plant-based supplement containing B-complex vitamins can improve bee health and increase colony performance. Vet. Med. 2021, 190, 105322–102332.
- Glavinic, U.; Stankovic, B.; Draskovic, V.; Stevanovic, J.; Petrovic, T.; Lakic, N.; Stanimirovic, Z. Dietary amino acid and vitamin complex protects honey bee from immunosuppression caused by Nosema ceranae. PLoS ONE 2017, 12, e0187726.
- DeGrandi-Hoffman, G.; Chen, Y.; Rivera, R.; Carroll, M.; Chambers, M.; Hidalgo, G.; de Jong, E.W. Honey bee colonies provided with natural forage have lower pathogen loads and higher overwinter survival than those fed protein supplements. Apidologie 2016, 47, 186–196. https://doi.org/10.1007/s13592-015-0386-6.
- Dolasevic, S.; Stevanovic, J.; Aleksic, N.; Glavinic, U.; Deletic, N.; Mladenovic, M.; Stanimirovic, Z. The effect of diet types on some quality characteristics of artificially reared Apis mellifera J. Apic. Res. 2020, 59, 115–123. https://doi.org/10.1080/00218839.2019.1673965.
- Ptaszyńska, A.A.; Borsuk, G.; Zdybicka-Barabas, A.; Cytryńska, M.; Małek, W. Are commercial probiotics and prebiotics effective in the treatment and prevention of honeybee nosemosis C? Res. 2016, 115, 397–406.
- Bartlett, L.J. Frontiers in effective control of problem parasites in beekeeping. J. Parasitol. Parasites Wildl. 2022, 17, 263–272. https://doi.org/10.1016/j.ijppaw.2022.03.003.
- Toomemaa, K. The synergistic effect of weak oxalic acid and thymol aqueous solutions on Varroa mites and honey bees. Apic. Res. 2019, 58, 37–52.
- Adamczyk, S.; Lázaro, R.; Pérez-Arquillué, C.; Conchello, P.; Herrera, A. Evaluation of residues of essential oil components in honey after different anti-Varroa treatments. Agric. Food Chem. 2005, 53, 10085–10090.
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. Invertebr. Pathol. 2010, 103, S96–S119. https://doi.org/10.1016/j.jip.2009.07.016.
- Sun, L.; Zhang, X.; Xu, S.; Hou, C.; Xu, J.; Zhao, D.; Chen, Y. Antiviral activities of a medicinal plant extract against sacbrood virus in honeybees. J. 2021, 18, 83–92.
- Tauber, J.P.; Collins, W.R.; Schwarz, R.S.; Chen, Y.; Grubbs, K.; Huang, Q.; Lopez, D.; Peterson, R.; Evans, J.D. Natural product medicines for honey bees: Perspective and protocols. Insects 2019, 10, 356–281. https://doi.org/10.3390/insects10100356.
- Santos, R.C.V.; Lopes, L.Q.S.; dos Alves, C.F.S.; Fausto, V.P.; Pizzutti, K.; Barboza, V.; de Souza, M.E.; Raffin, R.P.; Gomes, P.; Takamatsu, D.; et al. Antimicrobial activity of tea tree oil nanoparticles against American and European foulbrood diseases agents. Asia. Pac. Entomol. 2014, 17, 343–347. https://doi.org/10.1016/j.aspen.2014.02.003.
- Higes, M.; Nozal, M.J.; Alvaro, A.; Barrios, L.; Meana, A.; Martín-Hernández, R.; Bernal, J.L.; Bernal, J. The stability and effectiveness of fumagillin in controlling Nosema ceranae (Microsporidia) infection in honey bees (Apis mellifera) under laboratory and field conditions. Apidologie 2011, 42, 364–377.
- Bravo, J.; Carbonell, V.; Sepúlveda, B.; Delporte, C.; Valdovinos, C.E.; Martín-Hernández, R.; Higes, M. Antifungal activity of the essential oil obtained from Cryptocarya alba against infection in honey bees by Nosema ceranae. Invertebr. Pathol. 2017, 149, 141–147.
- Cilia, G.; Garrido, C.; Bonetto, M.; Tesoriero, D.; Nanetti, A. Effect of api-bioxal® and apiherb® treatments against Nosema ceranae infection in Apis mellifera investigated by two qPCR methods. Sci. 2020, 7, 125–136. https://doi.org/10.3390/vetsci7030125.
- Nanetti, A.; Ugolini, L.; Cilia, G.; Pagnotta, E.; Malaguti, L.; Cardaio, I.; Matteo, R.; Lazzeri, L. Seed meals from Brassica nigra and Eruca sativa control artificial Nosema ceranae infections in Apis mellifera. Microorganisms 2021, 9, 949–962. https://doi.org/10.3390/microorganisms9050949.
- Ugolini, L.; Cilia, G.; Pagnotta, E.; Malaguti, L.; Capano, V.; Guerra, I.; Zavatta, L.; Albertazzi, S.; Matteo, R.; Lazzeri, L.; et al. Glucosinolate bioactivation by Apis mellifera workers and its impact on Nosema ceranae infection at the colony level. Biomolecules 2021, 11, 1657–1678. https://doi.org/10.3390/biom11111657.
- Hasan, S. A review on nanoparticles: Their synthesis and types. J. Recent Sci 2015, 2277, 2502–2504.
- Sousa, F.; Ferreira, D.; Reis, S.; Costa, P. Current insights on antifungal therapy: Novel nanotechnology approaches for drug delivery systems and new drugs from natural sources. Pharmaceuticals 2020, 13, 248–277.
- Culha, M.; Kalay, Ş.; Sevim, E.; Pinarbaş, M.; Baş, Y.; Akpinar, R.; Karaoğlu, Ş.A. Biocidal properties of maltose reduced silver nanoparticles against American foulbrood diseases pathogens. Biometals 2017, 30, 893–902.
- Li, J.; Heerman, M.C.; Evans, J.D.; Rose, R.; Li, W.; Rodríguez-García, C.; DeGrandi-Hoffman, G.; Zhao, Y.; Huang, S.; Li, Z.; et al. Pollen reverses decreased lifespan, altered nutritional metabolism and suppressed immunity in honey bees (Apis mellifera) treated with antibiotics. Exp. Biol. 2019, 222, jeb202077. https://doi.org/10.1242/jeb.202077.
- Masry, S.H.D.; Taha, T.H.; Botros, W.A.; Mahfouz, H.; Al-Kahtani, S.N.; Ansari, M.J.; Hafez, E.E. Antimicrobial activity of camphor tree silver nano-particles against foulbrood diseases and finding out new strain of Serratia marcescens via DGGE-PCR, as a secondary infection on honeybee larvae. Saudi J. Biol. Sci. 2021, 28, 2067–2075. https://doi.org/10.1016/j.sjbs.2021.02.038.
- Dong, Z.; Wu, Q.; Long, J.; Lu, B.; Zheng, N.; Hu, C.; Chen, P.; Hu, N.; Lu, C.; Pan, M. Silver nanoparticles are effective in controlling microsporidia. Sci. Eng. C 2021, 125, 112106–112117.
- Potts, S.G.; Roberts, S.P.M.; Dean, R.; Marris, G.; Brown, M.A.; Jones, R.; Neumann, P.; Settele, J. Declines of managed honey bees and beekeepers in Europe. Apic. Res. 2010, 49, 15–22. https://doi.org/10.3896/IBRA.1.49.1.02.
- Li, G.; Zhao, H.; Liu, Z.; Wang, H.; Xu, B.; Guo, X. The wisdom of honeybee defenses against environmental stresses. Microbiol. 2018, 9, 722–736. https://doi.org/10.3389/fmicb.2018.00722.
- Tang, J.; Ma, C.; Shi, W.; Chen, X.; Liu, Z.; Wang, H. A National survey of managed honey bee colony winter losses (Apis mellifera) in China (2013–2017). Diversity 2020, 12, 318–331.
- Tawfik, A.I.; Ahmed, Z.H.; Abdel-Rahman, M.F.; Moustafa, A.M. Influence of winter feeding on colony development and the antioxidant system of the honey bee, Apis mellifera. Apic. Res. 2020, 59, 752–763. https://doi.org/10.1080/00218839.2020.1752456.
- Abera, A.; Yakob, H.; Yasin, G. Assessment of production system and constraints of bee keeping practices in damot gale woreda, wolaita zone, southern ethiopia. Hortic. For. 2016, 6, 109–114.
- Underwood, R.M.; Traver, B.E.; López-Uribe, M.M. Beekeeping management practices are associated with operation size and beekeepers’ philosophy towards in-hive chemicals. Insects 2019, 10, 10–22.
This entry is adapted from the peer-reviewed paper 10.3390/vetsci9050199
