Nanostructured titanium compounds have recently been applied in the design of gas sensors. Among titanium compounds, titanium oxides (TiO2) are the most frequently used in gas sensing devices. Very recently, the applicability of non-stoichiometric titanium oxide (TiO2−x)-based layers for the design of gas sensors was demonstrated. The most promising titanium compounds and hetero- and nano-structures based on these compounds are discussed and the possibility to tune the sensitivity and selectivity of titanium compound-based sensing layers is addressed.
- titanium dioxide (TiO)
- non-stoichiometric titanium oxide (TiO or TiO)
- magneli phases (TiO)
- gas and volatile organic compounds (VOCs) sensors
1. Structural Features and Physicochemical Properties of Stoichiometric and Non-Stoichiometric Titanium Oxides
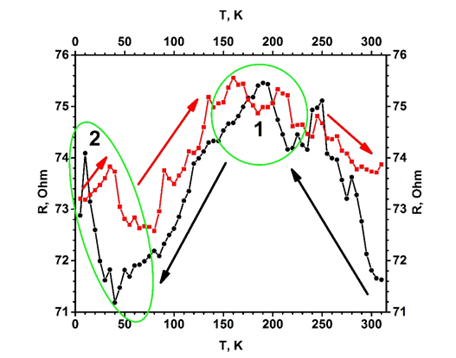
Figure 1. Temperature dependence of electrical resistance (R(T)) for the TiO2−x/TiO2(400 °C)-based hetero-structure. Temperature was changed in two ways (indicated by black and red arrows): (i) black cycles shows points measured by cooling down, (ii) red squares shows points by increasing temperature. Measurements were performed in vacuum using helium cryostat. Figure adapted from [3].
2. Pristine Titanium Oxide-Based Gas Sensors and Their Sensing Mechanisms
Table 1. Characteristics of titanium oxide-based sensors[34].
|
Sensing Material |
Working Temperature |
Gas Concentration |
Response Value (Ra/Rg) or ((ΔR/Rg) × 100%) |
Response Time |
Recovery Time |
Reference |
|
TiO2 (rutile), Ti8O15 and Ti9O17 mixture |
210 °C |
12.5–100 ppm (NH3) |
1–7% |
2 min |
8 min |
[35] |
|
TiOx-NiO |
250–350 °C |
100 ppm (H2) 100 ppm (NO2) 100 ppm (NH3) |
17 for H2 (250 °C) 16 for NO2 (250 °C) 4 for NH3 (250 °C) |
2 min |
2,3 min |
[36] |
|
β-Ti3O5 |
150 °C |
50 ppm (H2) |
11% |
- |
- |
[37] |
|
Ti3O5-TiO2 mixture |
25–180 °C |
105 ppm (H2O) 118 ppm (methanol) 53 ppm (ethanol) 18 ppm n-propanol 220 ppm (acetone) |
0.5–18% |
- |
4–35 s |
[3] |
|
TiO2-Ti6O |
150–450 °C |
2000 pm (H2) 20 ppm (NO2) 500 ppb (O3) 1.6 ppm (acetone) 80 ppm (NOx) |
2.9–348 |
8–21 s |
20–32 s |
[2] |
|
Ti3+-TiO2 |
RT |
100 ppm (CO) |
39% |
10 s |
30 s |
[38] |
|
TiO2 |
150 °C |
100 ppm (ethanol) |
75.4% |
155 s |
779 s |
[39] |
|
TiO2 |
270 °C |
500 ppm (acetone) |
9.19 |
10 s |
9 s |
[40] |
|
TiO2 |
350 °C |
400 ppm (ethanol) |
22.9 |
5 s |
7 s |
[41] |
|
TiO2 |
RT |
200 ppm (NH3) |
64 |
28 s |
24 s |
[42] |
|
α-Fe2O3-TiO2 |
325 °C |
100 ppm (ethanol) |
4 |
46 s |
16 s |
[43] |

Figure 2. Band diagram of TiO2/GO hetero-structure (a) before the formation of hetero-structure, (b) after the formation of hetero-structure, (c) when UV irradiation is applied, and (d) when UV irradiation is switched off. (‘e’ is an electron; ‘h’ is a hole) [34].
This entry is adapted from the peer-reviewed paper 10.3390/coatings12050699
References
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide Semiconductor Gas Sensors. Catal. Surv. Asia 2003, 7, 63–75.
- Maziarz, W.; Kusior, A.; Trenczek-Zajac, A. Nanostructured TiO2-Based Gas Sensors with Enhanced Sensitivity to Reducing Gases. Beilstein J. Nanotechnol. 2016, 7, 1718–1726.
- Ramanavicius, S.; Tereshchenko, A.; Karpicz, R.; Ratautaite, V.; Bubniene, U.; Maneikis, A.; Jagminas, A.; Ramanavicius, A. TiO2-x/TiO2-Structure Based ‘Self-Heated’ Sensor for the Determination of Some Reducing Gases. Sensors 2020, 20, 74.
- Tereshchenko, A.; Smyntyna, V.; Ramanavicius, A. Interaction Mechanism between TiO2 Nanostructures and Bovine Leukemia Virus Proteins in Photoluminescence-Based Immunosensors. RSC Adv. 2018, 8, 37740–37748.
- Tereshchenko, A.; Viter, R.; Konup, I.; Ivanitsa, V.; Geveliuk, S.; Ishkov, Y.; Smyntyna, V. TiO2 Optical Sensor for Amino Acid Detection. In Proceedings of the Biophotonics—Riga 2013, Riga, Latvia, 26–31 August 2013; Spigulis, J., Kuzmina, I., Eds.; SPIE: Washington, DC, USA, 2013; Volume 9032, pp. 186–190.
- Wunderlich, W.; Oekermann, T.; Miao, L.; Hue, N.T.; Tanemura, S.; Tanemura, M. ELECTRONIC PROPERTIES OF NANO-POROUS TiO 2- AND ZnO THIN FILMS- COMPARISON OF SIMULATIONS AND EXPERIMENTS. J. Ceram. Process. Res. 2004, 5, 343–354.
- Ohkoshi, S.; Tsunobuchi, Y.; Matsuda, T.; Hashimoto, K.; Namai, A.; Hakoe, F.; Tokoro, H. Synthesis of a Metal Oxide with a Room-Temperature Photoreversible Phase Transition. Nat. Chem. 2010, 2, 539–545.
- Yoshimatsu, K.; Sakata, O.; Ohtomo, A. Superconductivity in Ti4O7 and γ-Ti3O5 Films. Sci. Rep. 2017, 7, 12544.
- Marezio, M.; McWhan, D.B.; Dernier, P.D.; Remeika, J.P. Structural Aspects of the Metal-Insulator Transitions in Ti4O7. J. Solid State Chem. 1973, 6, 213–221.
- D’Angelo, A.M.; Webster, N.A.S. Evidence of Anatase Intergrowths Formed during Slow Cooling of Reduced Ilmenite. J. Appl. Crystallogr. 2018, 51, 185–192.
- Jayashree, S.; Ashokkumar, M. Switchable Intrinsic Defect Chemistry of Titania for Catalytic Applications. Catalysts 2018, 8, 601.
- Arūnas Jagminas; Simonas Ramanavičius; Vitalija Jasulaitiene; Mantas Šimėnas; Hydrothermal synthesis and characterization of nanostructured titanium monoxide films. RSC Advances 2019, 9, 40727-40735, 10.1039/c9ra08463k.
- Andersson, S.; Magnéli, A. Diskrete Titanoxydphasen Im Zusammensetzungsbereich TiO_1,75-TiO_1,90. Naturwissenschaften 1956, 43, 495–496.
- Liborio, L.; Mallia, G.; Harrison, N. Electronic Structure of the Ti4O7 Magneli Phase. Phys. Rev. B 2009, 79, 245133.
- Zhu, Q.; Peng, Y.; Lin, L.; Fan, C.-M.; Gao, G.-Q.; Wang, R.-X.; Xu, A.-W. Stable Blue TiO2−x Nanoparticles for Efficient Visible Light Photocatalysts. J. Mater. Chem. A 2014, 2, 4429–4437.
- Al-Hashem, M.; Akbar, S.; Morris, P. Role of Oxygen Vacancies in Nanostructured Metal-Oxide Gas Sensors: A Review. Sens. Actuators B Chem. 2019, 301, 126845.
- Ramanavicius, S.; Ramanavicius, A. Insights in the Application of Stoichiometric and Non-Stoichiometric Titanium Oxides for the Design of Sensors for the Determination of Gases and VOCs (TiO2−x and TinO2n−1 vs. TiO2). Sensors 2020, 20, 6833.
- Harada, S.; Tanaka, K.; Inui, H. Thermoelectric Properties and Crystallographic Shear Structures in Titanium Oxides of the Magnèli Phases Bandgap Engineering of Magnéli Phase TinO2n−1: Electron-Hole Self-Compensation. J. Appl. Phys. 2010, 108, 83703.
- Walsh, F.C.; Wills, R.G.A. The Continuing Development of Magnéli Phase Titanium Sub-Oxides and Ebonex® Electrodes. Electrochim. Acta 2010, 55, 6342–6351.
- Arūnas Jagminas; Arnas Naujokaitis; Paulius Gaigalas; Simonas Ramanavičius; Marija Kurtinaitienė; Romualdas Trusovas; Substrate Impact on the Structure and Electrocatalyst Properties of Molybdenum Disulfide for HER from Water. Metals 2020, 10, 1251, 10.3390/met10091251.
- Nakamura, I.; Negishi, N.; Kutsuna, S.; Ihara, T.; Sugihara, S.; Takeuchi, K. Role of Oxygen Vacancy in the Plasma-Treated TiO2 Photocatalyst with Visible Light Activity for NO Removal. J. Mol. Catal. A Chem. 2000, 161, 205–212.
- le Mercier, T.; Mariot, J.M.; Parent, P.; Fontaine, M.F.; Hague, C.F.; Quarton, M. Formation of Ti3+ Ions at the Surface of Laser-Irradiated Rutile. Appl. Surf. Sci. 1995, 86, 382–386.
- Zheng, Z.; Huang, B.; Meng, X.; Wang, J.; Wang, S.; Lou, Z.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y. Metallic Zinc- Assisted Synthesis of Ti3+ Self-Doped TiO2 with Tunable Phase Composition and Visible-Light Photocatalytic Activity. Chem. Commun. 2013, 49, 868–870.
- Hashimoto, S.; Tanaka, A. Alteration of Ti 2p XPS Spectrum for Titanium Oxide by Low-Energy Ar Ion Bombardment. Surf. Interface Anal. 2002, 34, 262–265.
- Hayfield, P.C.S. Development of a New Material: Monolithic Ti4O7 Ebonex Ceramic; Royal Society of Chemistry: London, UK, 2007; ISBN 184755069X.
- Åsbrink, S.; Magnéli, A. Crystal Structure Studies on Trititanium Pentoxide, Ti3O5. Acta Crystallogr. 1959, 12, 575–581.
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758.
- Wang, Y.; Du, G.; Liu, H.; Liu, D.; Qin, S.; Wang, N.; Hu, C.; Tao, X.; Jiao, J.; Wang, J.; et al. Nanostructured Sheets of Ti—O Nanobelts for Gas Sensing and Antibacterial Applications. Adv. Funct. Mater. 2008, 18, 1131–1137.
- Kimura, M.; Sakai, R.; Sato, S.; Fukawa, T.; Ikehara, T.; Maeda, R.; Mihara, T. Sensing of Vaporous Organic Compounds by TiO2 Porous Films Covered with Polythiophene Layers. Adv. Funct. Mater. 2012, 22, 469–476.
- Petruleviciene, M.; Juodkazyte, J.; Parvin, M.; Tereshchenko, A.; Ramanavicius, S.; Karpicz, R.; Samukaite-Bubniene, U.; Ramanavicius, A. Tuning the Photo-Luminescence Properties of WO3 Layers by the Adjustment of Layer Formation Conditions. Materials 2020, 13, 2814.
- Viter, R.; Tereshchenko, A.; Smyntyna, V.; Ogorodniichuk, J.; Starodub, N.; Yakimova, R.; Khranovskyy, V.; Ramanavicius, A. Toward Development of Optical Biosensors Based on Photoluminescence of TiO2 Nanoparticles for the Detection of Salmonella. Sens. Actuators B Chem. 2017, 252, 95–102.
- Haryński, Ł.; Grochowska, K.; Karczewski, J.; Ryl, J.; Siuzdak, K. Scalable Route toward Superior Photoresponse of UV-Laser-Treated TiO2 Nanotubes. ACS Appl. Mater. Interfaces 2020, 12, 3225–3235.
- Simonas Ramanavicius, Arunas Ramanavicius; Development of molecularly imprinted polymer based phase boundaries for sensors design (review). Advances in Colloid and Interface Science 2022, 305, 102693, 10.1016/j.cis.2022.102693.
- Simonas Ramanavicius; Arunas Jagminas; Arunas Ramanavicius; Gas Sensors Based on Titanium Oxides (Review). Coatings 2022, 12, 699, 10.3390/coatings12050699.
- M. Gardon; O. Monereo; S. Dosta; G. Vescio; A. Cirera; J.M. Guilemany; New procedures for building-up the active layer of gas sensors on flexible polymers. Surface and Coatings Technology 2013, 235, 848-852, 10.1016/j.surfcoat.2013.09.011.
- C Imawan; F Solzbacher; H Steffes; E Obermeier; TiOx-modified NiO thin films for H2 gas sensors: effects of TiOx-overlayer sputtering parameters. Sensors and Actuators B: Chemical 2000, 68, 184-188, 10.1016/s0925-4005(00)00427-5.
- Xiaolei Li; Ying Liu; Shiqing Ma; Jinwen Ye; Xiaoyan Zhang; Guangrui Wang; Yuchong Qiu; The synthesis and gas sensitivity of the beta-Ti3O5 powder: Experimental and DFT study. Journal of Alloys and Compounds 2015, 649, 939-948, 10.1016/j.jallcom.2015.07.094.
- Juan Su; Xiao-Xin Zou; Yong-Cun Zou; Guo-Dong Li; Pei-Pei Wang; Jie-Sheng Chen; Porous Titania with Heavily Self-Doped Ti3+ for Specific Sensing of CO at Room Temperature. Inorganic Chemistry 2013, 52, 5924-5930, 10.1021/ic400109j.
- Teena Gakhar; Arnab Hazra; Oxygen vacancy modulation of titania nanotubes by cathodic polarization and chemical reduction routes for efficient detection of volatile organic compounds. Nanoscale 2020, 12, 9082-9093, 10.1039/c9nr10795a.
- Sachin Navale; Z.B. Yang; Chenshitao Liu; P.J. Cao; V.B. Patil; N.S. Ramgir; Rajaram Mane; F.J. Stadler; Enhanced acetone sensing properties of titanium dioxide nanoparticles with a sub-ppm detection limit. Sensors and Actuators B: Chemical 2017, 255, 1701-1710, 10.1016/j.snb.2017.08.186.
- Xue Gao; Yanqiong Li; Wen Zeng; Caifeng Zhang; Yaoming Wei; Hydrothermal synthesis of agglomerating TiO2 nanoflowers and its gas sensing. Journal of Materials Science: Materials in Electronics 2017, 28, 18781-18786, 10.1007/s10854-017-7827-0.
- Neli Mintchevaac; Parthasarathy Srinivasanb; John Bosco Balaguru Rayappan; Aleksandr A.Kuchmizhakde; Stanislav Gurbatovde; Sergei Kulinich; Room-temperature gas sensing of laser-modified anatase TiO2 decorated with Au nanoparticles. Applied Surface Science 2019, 507, 145169, 10.1016/j.apsusc.2019.145169.
- Hui Mei; Shixiang Zhou; Mingyang Lu; Yong Zhao; Laifei Cheng; Construction of pine-branch-like alpha-Fe2O3/TiO2 hierarchical heterostructure for gas sensing. Ceramics International 2020, 46, 18675-18682, 10.1016/j.ceramint.2020.04.181.
- Hsu, K.C.; Fang, T.H.; Hsiao, Y.J.; Wu, P.C. Response and Characteristics of TiO2/Perovskite Heterojunctions for CO Gas Sensors. J. Alloys Compd. 2019, 794, 576–584.
- Avansi, W.; Catto, A.C.; da Silva, L.F.; Fiorido, T.; Bernardini, S.; Mastelaro, V.R.; Aguir, K.; Arenal, R. One-Dimensional V2O5/TiO2 Heterostructures for Chemiresistive Ozone Sensors. ACS Appl. Nano Mater. 2019, 2, 4756–4764.
- Chen, K.; Chen, S.; Pi, M.; Zhang, D. SnO2 Nanoparticles/TiO2 Nanofibers Heterostructures: In Situ Fabrication and Enhanced Gas Sensing Performance. Solid-State Electron. 2019, 157, 42–47.
- Yu, Q.; Zhu, J.; Xu, Z.; Huang, X. Facile Synthesis of α-Fe2O3@SnO2 Core–Shell Heterostructure Nanotubes for High Performance Gas Sensors. Sens. Actuators B Chem. 2015, 213, 27–34.
- Seekaew, Y.; Wisitsoraat, A.; Phokharatkul, D.; Wongchoosuk, C. Room Temperature Toluene Gas Sensor Based on TiO2 Nanoparticles Decorated 3D Graphene-Carbon Nanotube Nanostructures. Sens. Actuators B Chem. 2019, 279, 69–78.
- Stratakis, E.; Savva, K.; Konios, D.; Petridis, C.; Kymakis, E. Improving the Efficiency of Organic Photovoltaics by Tuning the Work Function of Graphene Oxide Hole Transporting Layers. Nanoscale 2014, 6, 6925–6931.
- Chen, C.; Cai, W.; Long, M.; Zhou, B.; Wu, Y.; Wu, D.; Feng, Y. Synthesis of Visible-Light Responsive Graphene Oxide/TiO2 Composites with p/n Heterojunction. ACS Nano 2010, 4, 6425–6432.
- Lightcap, I.V.; Kosel, T.H.; Kamat, P. Anchoring Semiconductor and Metal Nanoparticles on a Two-Dimensional Catalyst Mat. Storing and Shuttling Electrons with Reduced Graphene Oxide. Nano Lett. 2010, 10, 577–583.
- Lam, K.C.; Huang, B.; Shi, S.-Q. Room-Temperature Methane Gas Sensing Properties Based on in Situ Reduced Graphene Oxide Incorporated with Tin Dioxide. J. Mater. Chem. A 2017, 5, 11131–11142.
- Ye, Z.; Tai, H.; Xie, T.; Yuan, Z.; Liu, C.; Jiang, Y. Room Temperature Formaldehyde Sensor with Enhanced Performance Based on Reduced Graphene Oxide/Titanium Dioxide. Sens. Actuators B Chem. 2016, 223, 149–156.
- Buchsteiner, A.; Lerf, A.; Pieper, J. Water Dynamics in Graphite Oxide Investigated with Neutron Scattering. J. Phys. Chem. B 2006, 110, 22328–22338.
- Phan, D.T.; Chung, G.S. Effects of Rapid Thermal Annealing on Humidity Sensor Based on Graphene Oxide Thin Films. Sens. Actuators B Chem. 2015, 220, 1050–1055.
- Wang, P.; Zhai, Y.; Wang, D.; Dong, S. Synthesis of Reduced Graphene Oxide-Anatase TiO2 Nanocomposite and Its Improved Photo-Induced Charge Transfer Properties. Nanoscale 2011, 3, 1640–1645.
- Cui, S.; Wen, Z.; Huang, X.; Chang, J.; Chen, J. Stabilizing MoS2 Nanosheets through SnO2 Nanocrystal Decoration for High-Performance Gas Sensing in Air. Small 2015, 11, 2305–2313.
- Lee, E.; Lee, D.; Yoon, J.; Yin, Y.; Lee, Y.N.; Uprety, S.; Yoon, Y.S.; Kim, D.-J. Enhanced Gas-Sensing Performance of GO/TiO2 Composite by Photocatalysis. Sensors 2018, 18, 3334.
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonavita, A.; Bonyani, M.; Leonardi, S.G.; Neri, G. Synthesis, Characterization and Gas Sensing Properties of α-Fe2O3 Core–Shell Nanocomposites. Nanomaterials 2015, 5, 737–749.
- Mosadegh Sedghi, S.; Mortazavi, Y.; Khodadadi, A. Low Temperature CO and CH4 Dual Selective Gas Sensor Using SnO2 Quantum Dots Prepared by Sonochemical Method. Sens. Actuators B Chem. 2010, 145, 7–12.
- Rieu, M.; Camara, M.; Tournier, G.; Viricelle, J.P.; Pijolat, C.; de Rooij, N.F.; Briand, D. Fully Inkjet Printed SnO2 Gas Sensor on Plastic Substrate. Sens. Actuators B Chem. 2016, 236, 1091–1097.
- Chung, F.C.; Wu, R.J.; Cheng, F.C. Fabrication of a 2 Core–Shell Structure for Gaseous Formaldehyde Sensing at Room Temperature. Sens. Actuators B Chem. 2014, 190, 1–7.
- Chen, G.; Ji, S.; Li, H.; Kang, X.; Chang, S.; Wang, Y.; Yu, G.; Lu, J.; Claverie, J.; Sang, Y.; et al. High-Energy Faceted SnO2-Coated TiO2 Nanobelt Heterostructure for Near-Ambient Temperature-Responsive Ethanol Sensor. ACS Appl. Mater. Interfaces 2015, 7, 24950–24956.
- Li, F.; Gao, X.; Wang, R.; Zhang, T.; Lu, G. Study on TiO2-SnO2 Core-Shell Heterostructure Nanofibers with Different Work Function and Its Application in Gas Sensor. Sens. Actuators B Chem. 2017, 248, 812–819.
- Zeng, W.; Liu, T.; Wang, Z. UV Light Activation of TiO2 Doped SnO2 Thick Film for Sensing Ethanol at Room Temperature. Mater. Trans. 2010, 51, 243–245.
- Lee, H.C.; Hwang, W.S. Substrate Effects on the Oxygen Gas Sensing Properties of SnO2/TiO2 Thin Films. Appl. Surf. Sci. 2006, 253, 1889–1897.
- Lee, J.H.; Mirzaei, A.; Kim, J.H.; Kim, J.Y.; Nasriddinov, A.F.; Rumyantseva, M.N.; Kim, H.W.; Kim, S.S. Gas-Sensing Behaviors of TiO2-Layer-Modified SnO2 Quantum Dots in Self-Heating Mode and Effects of the TiO2 Layer. Sens. Actuators B Chem. 2020, 310, 127870.
- Ng, S.; Prášek, J.; Zazpe, R.; Pytlíček, Z.; Spotz, Z.; Pereira, J.R.; Michalička, J.; Přikryl, J.; Krbal, M.; Sopha, H.; et al. Atomic Layer Deposition of SnO2-Coated Anodic One-Dimensional TiO2 Nanotube Layers for Low Concentration NO2 Sensing. ACS Appl. Mater. Interfaces 2020, 12, 33386–33396.
- Song, Z.; Wei, Z.; Wang, B.; Luo, Z.; Xu, S.; Zhang, W.; Yu, H.; Li, M.; Huang, Z.; Zang, J.; et al. Sensitive Room-Temperature H2S Gas Sensors Employing SnO2 Quantum Wire/Reduced Graphene Oxide Nanocomposites. Chem. Mater. 2016, 28, 1205–1212.
- Nasriddinov, A.; Rumyantseva, M.; Marikutsa, A.; Gaskov, A.; Lee, J.-H.; Kim, J.-H.; Kim, J.-Y.; Kim, S.S.; Kim, H.W. Sub-Ppm Formaldehyde Detection by n-n TiO2@SnO2 Nanocomposites. Sensors 2019, 19, 3182.
- Shaposhnik, D.; Pavelko, R.; Llobet, E.; Gispert-Guirado, F.; Vilanova, X. Hydrogen Sensors on the Basis of SnO2–TiO2 Systems. Sens. Actuators B: Chem. 2012, 174, 527–534.
- Plecenik, T.; Moško, M.; Haidry, A.A.; Ďurina, P.; Truchlý, M.; Grančič, B.; Gregor, M.; Roch, T.; Satrapinskyy, L.; Mošková, A.; et al. Fast Highly-Sensitive Room-Temperature Semiconductor Gas Sensor Based on the Nanoscale Pt–TiO2–Pt Sandwich. Sens. Actuators B Chem. 2015, 207, 351–361.
