The repair of infected bone defects (IBDs) is still a great challenge in clinic. A successful treatment for IBDs should simultaneously resolve both infection control and bone defect repair. Hydrogels are water-swollen hydrophilic materials that maintain a distinct three-dimensional structure, helping load various antibacterial drugs and biomolecules. Hybrid hydrogels may potentially possess antibacterial ability and osteogenic activity.
Bone grafts used to treat IBDs should act as osteoinductive bone substitutes and antimicrobial carriers [
12]. Autologous bone, also known as autograft, is still regarded as the clinical “gold standard” for bone repair. However, there are several limitations to autogenous grafting associated with the harvesting process. The shortcomings include morbidity of the donor site, increased blood loss, and longer operating times [
24]. Furthermore, the allograft is a limited supply of autologous bone substitutes because of the high expenses and dangers of viral transmission [
24,
25,
26]. Fortunately, bone substitutes or synthetic grafts are intended to overcome the drawbacks of autologous and allogeneic bone grafts. When used to restore contaminated bone tissue, bone grafts should ideally inhibit local bacterial growth. Simultaneously, it should stimulate cellular infiltration and immunomodulatory effects in host reparative cells [
27,
28].
Fabrication of biomedical materials with good antimicrobial and osteogenic activities is critical for promoting the repair effects of bone substitutes on IBDs [
29]. Several common materials have been extensively used in bone tissue engineering, including nanofibrous materials, coatings, and hydrogels [
30]. In particular, hydrogels have porous network structures and good biocompatibility to mimic the extracellular matrix (ECM) [
31]. As a distinct class of soft materials, hydrogels are composed of hydrophilic networks that can maintain moisture. Hydrogel is a suitable candidate to be used as carrier materials for cells or bone growth to facilitate growth factors released and can be easily loaded with antibacterial agents [
32]. Hydrogels can be fabricated from polymer chains connected by physical interactions or chemical bonds, and varying crosslinking methods and degrees can easily control the degradation rate, porosity, or release profile [
33]. Additionally, hydrogels can self-assemble with self-complementary amphiphilic peptides by gelation. Furthermore, they can be tailored to meet the optimum geometry for implantation or injection [
34]. Hydrogels are appealing therapeutic delivery materials, presenting the great potential to encapsulate agents in the water-swollen network [
35]. Additionally, some types of hydrogels have inherent antibacterial properties, such as chitosan (CS) and polyethyleneimine (PEI) [
32,
36,
37]. So hydrogels are scaffolds that have been widely researched as a potential alternative material for antibacterial tissue engineering.
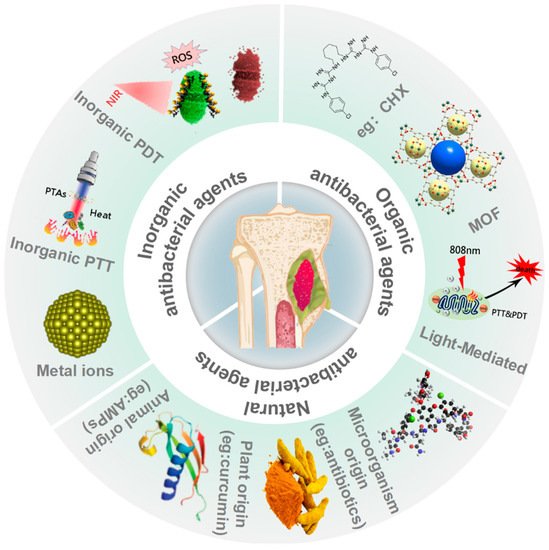
Antibacterial agents can be classified into three types: inorganic antibacterial agents, organic antibacterial agents, and natural antibacterial agents based on their composition, source, and nature. Additionally, each type is sorted into different categories, as summarized in Figure 1.
Antibacterial agents administered systemically have a lot of drawbacks, such as low concentrations in the infected area and side effects. In comparison, local delivery of antimicrobial agents may offer appropriate antibacterial dosages [
38]. Sustainable local delivery of antibacterial agents via a delivery carrier avoids many disadvantages of systemic side effects. Due to the excellent water content, great bioactivity, and convenience of drug-loading, hydrogels have been extensively researched as drug carriers for targeted delivery [
39]. Antibacterial agents can be used in conjunction with hydrogels to slow down the kinetics of drug release and deliver the medication to the target site. Moreover, the hydrogels’ degradation rate can also be controlled, providing this material system the characteristics of a prolonged-release cycle and reducing administration dosage [
40,
41]. Therefore, hydrogels can encapsulate agents or agent-loaded nano-/microcarriers to provide sustained localized antimicrobial drug release for excellent antibacterial and bone repair performance [
42].
2. Hybrid Hydrogels with Inorganic Antibacterial Agents for Infected Bone Repair
Inorganic antibacterial agents are classified based on their modes of action: metal ion elements (e.g., silver (Ag), gold (Au), copper (Cu), zinc (Zn)), and inorganic light-mediated antibacterial materials (e.g., reduced graphene oxide (rGO), carbon-based nanomaterial, titanium dioxide (TiO
2), zinc oxide (ZnO) [
43]. Light-mediated antibacterial activity can be achieved through photothermal therapy (PTT), photodynamic therapy (PDT), and sunlight-mediated antibacterial treatments [
44]. There are few studies on sunlight-activated nanomaterials to date, so this review will focus on the PTT and PDT related inorganic light-mediated antibacterial agents.
2.1. Hydrogels with Metal Nanomaterials
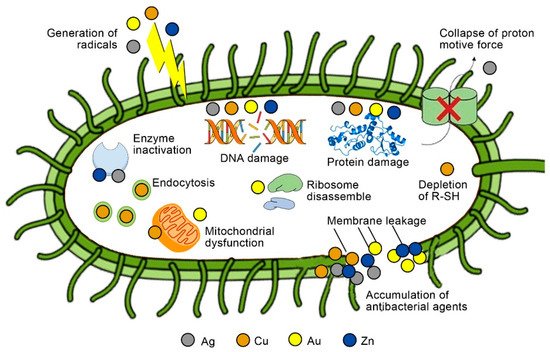
The antibacterial action of nanoparticles is achieved in a number of ways. Several factors, such as the released metal ions and the physicochemical characterization of nanoparticles, may lead to membrane disruption or cell wall penetration, which can contribute to nanoparticles’ antibacterial activity [
45,
46]. It has been shown that metallic nanoparticles (as in silver, gold, copper, and titanium) have significant antibacterial activity [
47,
48,
49]. The mechanisms of inorganic antibacterial agents of several metal ions are illustrated in
Figure 2.
Figure 2. Possible antibacterial mechanisms for inorganic antibacterial agents of Ag, Cu, Au, and Zn. R-SH, sulfhydryls (Reprinted with permission from Ref. [
50] Copyright 2021 Elsevier).
Among the several metal nanomaterials applied in antibacterial therapy, silver nanoparticles (AgNPs) are the most extensively investigated antibacterial nanoagent with a broad antibacterial spectrum [
51,
52]. AgNPs are typically assumed to perform antibacterially by attaching to the cell wall and membrane, and then destroying the structures and biomolecules within the cell with AgNPs and silver ions [
53,
54,
55]. At the same time, AgNPs can promote bone formation and accelerate the rehabilitation of injured tissues. Mahmood M et al. demonstrated that AgNPs could regulate many osteogenic genes related to bone growth [
56]. Han et al. described a method to synthesize AgNPs-loaded hydrogels using gelatin (Gel) as a stabilizing agent in a simple way under sunlight, which improved the survivability and proliferation of osteoblasts on the hydrogels for bone fracture treatment [
57].
Gold nanoparticles (GNPs) are also gaining immense attention since their antimicrobial activity has been reported [
58]. After intracellular uptake, GNPs have been demonstrated to stimulate osteogenic differentiation and mineralization in cells [
59,
60]. For example, Zhang et al. prepared PEG-hydrogels with GNPs of 4 nm, 18 nm, and 45 nm in size. The results indicated that hydrogels containing GNPs of 45 nm could efficiently induce bone regeneration in vivo by increasing the osteogenic gene expression, mineralization, and alkaline phosphatase (ALP) activity [
61]. In another case, Lee D et al. designed a hydrogel that tyramine (Ty) bound with the Gel backbone (Gel-Ty) containing GNPs attached to N-acetyl cysteine (NAC) (Gel-Ty/G-NAC) for effective bone regeneration [
62]. Furthermore, GNPs can be utilized for PTT to treat tumors when exposed to near-infrared light [
63]. In addition, copper nanoparticles show excellent antibacterial ability for both Gram-positive bacteria (GPB) and Gram-negative bacteria (GNB) [
64]. For example, Dai Q et al. fabricated a unique 3D-printed Ty-modified Gel/silk fibroin (SF)/copper (Cu)-doped bioactive glass (BG) hydrogel [
65]. The hydrogel with 1 wt% Cu-BG can effectively modulate osteogenesis and vascularization’s spatiotemporal coupling.
Like antibiotics, prolonged usage of AgNPs results in the development of multidrug-resistant microorganisms [
66]. Unfortunately, inorganic nanoparticles are difficult to biodegrade in vivo. So the toxicity of inorganic nanoparticles should be reduced by surface modification.
2.2. Light-Mediated Inorganic Antibacterial Hydrogels
In comparison to traditional antibiotics, PTT would not induce bacterial resistance [
67]. Aside from metal NPs, various photothermal agents (PTAs) have been successfully used in the antimicrobial field. PTAs can convert light into heat, resulting in rupture of the cell membrane, protein denaturation, and microbial death [
68]. PTT has demonstrated significant promise in antibacterial and bone regeneration treatment due to the rapid development of different PTAs. The inorganic nanomaterials with PPT abilities include metal nanomaterials (Au, Pt), carbon-based nanomaterials (graphene, fullerene, rGO), black phosphorus (BP), and other metal oxide nanoparticles [
44,
69,
70]. Unlike PTT, PDT generates reactive oxygen species (ROS) to generate cytotoxicity. Three elements are required for PDT: light, molecular oxygen, and photosensitizers (PSs). When the PSs are irradiated with light whose wavelength meets the PSs’ absorption, singlet oxygen (
1O
2), hydroxyl radicals, or oxygen-free radicals can be produced. These radicals can destroy cell membranes and DNA molecules [
71].
Nanoparticles with photothermal and photodynamic ability have recently received much attention as a potential treatment for bacterial infections and bone healing. Geng et al. developed a multifunctional biodegradable gelatin/methacrylate anhydride (GelMA) hydrogel by controlling the surface charge and preventing the positive- and negative- charged carbon quantum dots (CQD)from aggregating [
72]. They deposited positively charged carbon quantum dots (p-CQDs) on the surface of tungsten disulfide (WS
2) nanosheets. Additionally, Geng et al. incorporated (p-CQDs)/WS
2 with antimicrobial effects and negatively charged CQDs (n-CQDs) with bone induction ability in GelMA hydrogels. Not only can the hydrogels effectively kill multidrug-resistant bacteria (MDR), but they also considerably accelerate bone regeneration. Graphene, a typical carbon-based nanomaterial, has been extensively investigated for its ability to stimulate bone formation through interaction with osteoprogenitors and other skeletal progenitors. rGO is the product of treating graphene oxide (GO) under thermal, chemical, or UV [
73]. In addition to improving mechanical properties, graphene family materials uniformly dispersed into polymers to produce materials can also promote cell proliferation and differentiation, hence facilitating bone regeneration [
74]. Wang et al. fabricated the NIR light-responsive, rGO-loaded CS hydrogel films by electrodeposition [
75]. The histological and radiological examination revealed that the films promoted bone regeneration in calvarial defect osteoporotic models. Li et al. developed hybrid hydrogels containing gelatin methacrylate, β-cyclodextrin-modified rGO, and acryloyl-β-cyclodextrin for infected skull defects [
76]. These hydrogels exhibited ideal antibacterial photothermal properties, as well as unswelling and mechanical properties.
The difficult biodegradation of GO limits its biomedical applications, particularly in vivo [
77]. Conversely, BP can degrade in aqueous conditions, generating harmless phosphates and phosphonates that promote biomineralization and regulate osteogenesis [
78,
79]. As a recently emerged 2D nanomaterial, BP has stimulated widespread research interest. For example, Miao et al. reported that the BP/Gel hydrogel could promote osteogenesis in vitro without osteoinductive factors. In the Sprague Dawley rat model, they also found considerable newborn cranial bone tissue growth [
80].
The human body is capable of withstanding high heat for a brief period of time, but normal cells in the surrounding area could be damaged [
81,
82]. The NIR light frequently employed for PTT therapy has a limited penetration depth [
83]. In comparison to NIR-I light (650–1000 nm), the NIR-II window (1000–1700 nm) exhibits a greater penetration depth in tissue and lower energy attenuation [
84,
85]. Additionally, the combination of PDT and PTT can significantly enhance the antibacterial efficiency of phototherapy. As shown in
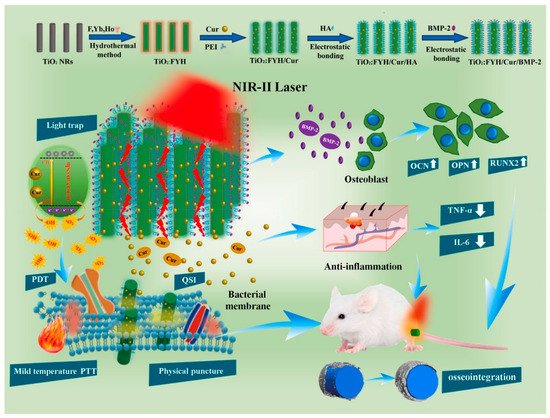
Figure 3, Zhang et al. designed a NIR-II phototherapy system using ytterbium (Yb), erbium (Er), and holmium(Ho) co-doped TiO
2 nanorods (TiO
2 NRs) (TiO
2:FYH)/curcumin (Cur)/hyaluronic acid (HA)/bone morphogenetic protein-2 (BMP-2) [
86]. It had antibiofilm, anti-inflammatory, and osteogenic capabilities in vitro and in vivo. The temperature increased to 47 °C when the 1060 nm laser was used, which was higher by about 7.2 °C than that of the 808 nm laser in the rabbit femur. Furthermore, the system exhibited great antibiofilm capability in the rabbit femur when irradiated with a 1060 nm laser, while numerous microorganisms lived when irradiated with an 808 nm laser. Then, on a titanium bone implant, they constructed a NIR-II-triggered nano-platform made of Yb and Er-doped TiO
2 nano-shovel (TiO
2@UCN)/quercetin (Qr)/L-arginine (LA) [
87]. When irradiated with a 1060 nm laser, the nanoplatform can eradicate biofilms on the titanium implants at 45 °C. Furthermore, the nano-platform enhanced revascularization and osteogenic differentiation, reduced inflammation, and promoted the generation of bone structures.
Figure 3. Schematic illustration of the crafting process of the TiO
2: FYH/Cur/BMP-2 NRs on Ti implant towards biofilm elimination, anti-inflammation, and bone regeneration. OCN, osteocalcin; OPN, osteopontin; RUNX2, runt-related transcription factor 2; QSI, quorum-sensing inhibitors; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6 (Reprinted with permission from Ref. [
86]. Copyright 2021 Elsevier).
High temperatures and
1O
2 from the phototherapy could easily destroy adjacent tissues, such as the periosteum and blood vessels [
88]. PSs can also be developed to be activated by enzyme-mediated luminescence techniques in addition to external sources of excitation, allowing them to address depth constraints [
89]. Developing near-infrared light-triggered nanomaterials with extremely prolonged luminescence lifetimes, allowing for continuous activation of PSs for phototherapy, may provide another way to avoid external light irradiation [
70].
3. Hybrid Hydrogels with Organic Antibacterial Agents for Infected Bone Repair
Organic antibacterial agents including glutaraldehyde, quaternary ammonium salt compounds, and chlorhexidine (CHX), have been extensively studied [
90,
91,
92]. Metal-organic frameworks (MOFs) are effective against bacteria. MOFs usually refer to composites with a network structure by the self-assembly of metal ions and organic ligands. In comparison to traditional bactericidal materials, MOFs exhibit larger specific surface areas, more adjustable pore structures, and controllable ion release rates. As a result, MOFs have a promising future in infected bone regeneration [
93]. In addition to the inorganic photothermal materials mentioned above, organic photothermal agents have received much attention in recent years.
3.1. Hybrid Hydrogels with Organic Antibacterial Agents
Most inorganic antibacterial agents appear in the form of metal ions to kill GNB, whereas GPB are sensitive to organic antibacterial compounds via organelle modification and disruption of metabolic processes [
50]. There are many organic antibacterial agents, such as CHX, organic acids, phenols, and quaternary ammonium compounds [
94,
95].
Quaternary ammonium salts (QAS) are important synthetic organic antimicrobials with a broad antimicrobial spectrum. QASs’ hydrophobic and ionic interactions with biological membranes damage microorganisms’ barriers [
96,
97]. For example, Lin et al. used quaternary ammonium chitosan (QTS) as a liquid phase in conjunction with calcium silicate (CaSi) powder to form cement [
98]. When considering the osteogenic capacity, the antibacterial ability, and the setting time, the results revealed that CaSi cement with1% QTS might be a promising choice for bone regeneration.
Like QAS, CHX is commonly applied by healthcare personnel for general disinfection and hand hygiene [
99]. CHX is a broad-spectrum antimicrobial material that inhibits the formation of biofilms and GPB/GNB growth, particularly against E. faecalis [
100]. The antibacterial effect of CHX is mediated by the cation’s electrostatic interaction with the negatively charged portions of the bacterial surface, interfering with physiological activities and osmotic regulation in bacteria [
101]. Xu L et al. developed a novel injectable hydrogel composed of nanohydroxyapatite particles and CHX (nHA/CHX) loaded in gellan gum (GG), which has the potential to enhance the repair of IBDs [
102]. Bacteria counts were considerably lower in the surrounding bone tissue of rats treated with surgical debridement and GG/nHA/CHX transplantation than in the control group. Additionally, at 4 and 8 weeks, rats in the hydrogel group demonstrated considerably abundant new bone formation compared to the control group.
The antibacterial actions of the various organic antibacterial agents encompass a variety of distinct methods, including breaking down cell membranes or oxidizing the proteins and amino acids inside bacteria [
103]. However, organic antimicrobials have some limitations in biodegradability, stability, and lifetimes [
104]. For overcoming these problems, MOFs may be the solution.
3.2. Hybrid Hydrogels with Metal-Organic Frameworks
Due to the rapid rate of evolution of bacteria, the resistance of bacteria to many organic antimicrobial agents is increasing, which is an urgent problem in the healthcare system [
105]. MOFs have attracted substantial attention recently as an innovative and fast-evolving group of organic-inorganic hybrid materials [
106]. The majority of MOFs display antimicrobial properties by decomposing metal-ligand bonds and releasing ligands or metal ions into the bacteria. Additionally, they can be used as medication carriers through the adsorption or binding of medicines to their surfaces [
107,
108]. Various metal ions have been shown to have different effects on osteogenesis and bone mineralization, and their mechanisms of action have also been investigated. As a result, it was established that MOFs enhance osteogenic differentiation in vitro. In vivo studies were less common, which means that the application of MOFs for orthopaedic implants is just starting to be investigated [
109].
As an essential member of MOFs, zeolitic imidazolate frameworks-8 (ZIF-8) is a monocrystal constructed of Zn
2+ that connects to each other [
110]. Recently, Zhang’s study generated antibacterial ZIF-8 using the diethanolamine template and solvent techniques [
111]. The ZIF-8 synthesized in these two techniques exhibits remarkable antibacterial activity and is biocompatible at low concentrations. Taking advantage of its prolonged release of Zn
2+, which is essential in bone regeneration, revascularization, and antimicrobial activities, ZIF-8 has the promise to be applied as a modification material in bone tissue engineering. When applied to rat bone marrow stromal cells (rBMSCs), ZIF-8 activated the extracellular-signal-regulated kinase (ERK) pathway primarily, and eventually activated the classical mitogen-activated protein kinase (MAPK) signaling and promoted osteogenesis. [
112]. For example, Liu et al. designed ZIF-8 nanoparticles (ZIF-8 NPs) functionalized catechol-chitosan (CA-CS) hydrogels (CA-CS/Z) to guarantee adequate blood supply, maintain the stabilization of the bone transplant environment, enhance osteogenesis, and promote bone regeneration (
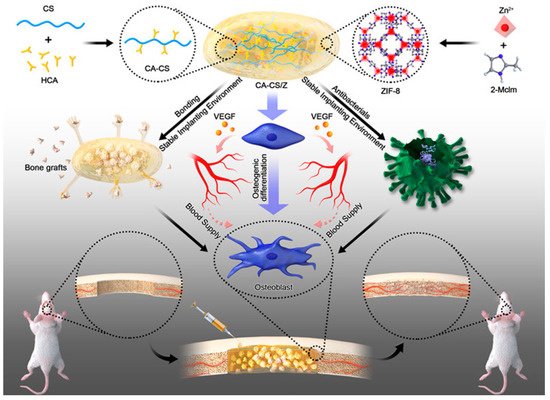
Figure 4) [
113]. The hydrogel demonstrated satisfactory adhesion and antimicrobial activities. ZIF-8 discharged from hydrogels may also increase the release and formation of osteocalcin, collagen I, and ALP, hence enhancing rBMSCs’ osteogenic differentiation.
Figure 4. Scheme of the fabrication of CA-CS/Z hydrogels with acceptable adhesion properties and antibacterial properties, enhancing the stability of the implanting environment after bone transplantation. HCA, hydrocaffeic acid; 2-Mclm, 2-methylimidazole; VEGF, vascular endothelial growth factor (Reprinted with permission from Ref. [
113]. Copyright 2020 American Chemical Society).
Nonetheless, excessive metal ions produced by MOFs may be toxic to human cells [
51,
114]. Numerous institutions are researching ways to improve the stability of metal ions as a solution to this issue. Zheng et al. fabricated a nanoplate with a gallic-acid-magnesium-based MOFs (Mg-MOF) core and a biodegradable calcium phosphate (CaP) shell [
115]. With the shell in place, the core was less susceptible to degradation, and the bioactive components contained within were more likely to reach a prolonged release under low-pH conditions stimulated by cytokine interleukin-4 (IL4). Then, IL4-MOF@CaP was integrated into collagen (Col) to create a biodegradable scaffold with significant bone regeneration. In addition to being composed of metal ions with antibacterial properties to exert antibacterial effects, MOFs can be loaded with various antibacterial agents as carriers [
116]. For instance, Huang et al. successfully constructed an intelligent and long-lasting agent carrier of MOFs(HKUST-1)@carboxymethyl chitosan (HKUST-1@CMCS) [
117]. These results indicated that dimethyl fumarate-loaded carrier had enhanced and long-lasting antibacterial action.
3.3. Light-Mediated Organic Antibacterial Hydrogels
Organic photothermal agents are categorized into two types: organic nanoparticles (such as porphyrin–lipid conjugate porphysome and organic semiconducting polymer nanoparticles) and organic dye molecules (such as indocyanine green (ICG), IR820, IR780) [
70,
118,
119]. These photothermal conversion materials are biodegradable but easily photodegradable or photobleached [
120].
Kuang et al. developed an injectable multifunctional hydrogel for NIR-triggered release for bone regeneration. This hydrogel consisted of poly (dimethylaminoethyl methacrylate-co-2-hydroxyethyl methacrylate)-coordinated situ-generated CaP nanoparticle (ICPN) (poly (DMAEMA-co-HEMA)/ICPN) (DHCP) hydrogel loaded with poly (N-acryloyl glycinamide-co-acrylamide) (PNAm)-ICG- parathyroid hormone (PTH) microspheres (PIP MSs) [
121]. Through the photothermal activity of ICG and the thermal polymerization of PNAm, the temperature was rapidly raised, so that PTH can be released accurately and controlled. The injectable NIR (808nm)-light-responsive hydrogel may stimulate osteoblast and osteoclast activity simultaneously and repair cranial defects successfully.
Additionally, served as PTAs, Polydopamine (PDA) exhibits excellent photothermal conversion and adhesion abilities [
121,
122]. Luo et al. combined immobilized cisplatin with PDA-modified nano-hydroxyapatite (HA) in an injectable hydrogel composed of oxidized sodium alginate and CS. In animals, the hydrogel had photothermal anticancer effects and facilitated the growth of new bone structures [
123]. Yao et al. prepared HA, PDA, and carboxymethyl chitosan (CMCS) composite scaffolds [
124]. In vitro, the scaffolds with PDA may stimulate higher BMSCs’ osteogenic differentiation than scaffolds lacking PDA. Additionally, the effect of the photothermal process on the osteogenic differentiation was not affected.
The disadvantage of organic photothermic agents is their susceptibility to photobleaching. Not only are conventional organic NIR-absorbing compounds difficult to synthesize, but they are also prone to photobleaching when exposed to light. These disadvantages result in increased costs and the possibility of performance degradation in PTT. Organic photothermal agents must therefore be modified or packaged to maintain their photothermal capabilities [
125].