1. Plasma
Plasma is a partially or fully ionized gas that can be considered the fourth state of matter based on thermodynamic considerations. It is characterized by the presence of neutral and electrically charged particles such as atoms, radicals free electrons, ions, and photons (including UV photons) [
66,
67]. Plasma is generally macroscopically neutral in terms of electrostatic charge. Plasmas can be produced over a wide range of pressures and temperatures, but two basic types are known: equilibrium (thermal) and non-equilibrium (cold) plasmas. In the former, the kinetic energy (temperature) of atoms and electrons is in thermodynamic equilibrium, therefore they cannot be used for the surface modification of heat-sensitive materials, while in the latter, the temperature of electrons is significantly higher than that of heavy particles. Plasmas and their applications are described in detail in several excellent review papers [
68,
69,
70,
71].
In cold plasma the heavy particles are at or at near room temperature, while the electron temperatures can be higher by orders of magnitude. High electron temperatures lead to high chemical reactivity, so cold plasma is suitable for surface modification of solids [
72,
73], even if they have otherwise low chemical reactivity in the conventional sense. Cold plasma can be formed and maintained in several ways, even thermally, but due to its efficiency, it is advisable to use an electrostatic or electromagnetic field for this purpose. Cold plasma treatment can be performed under reduced pressure or even at atmospheric pressure. Although both are viable pathways and the former has a larger scientific literature, the latter has significant advantages. Atmospheric methods are extensively developed and used; therefore, we will focus on describing them below.
1.1. Atmospheric Plasma Treatment Applications
Atmospheric plasmas have a wide range of applications. Plasmas are widely used in treatments involving hazardous or undesirable gases or wastes [
74]. Volatile organic compounds (VOCs) or inorganic gases such as nitrogen oxides (NO
x) or sulfur dioxide, pollute the environment when released into the atmosphere. These toxic molecules can collide with the reactive particles in the plasma and form free radicals that can assemble into harmless molecules. Plasma can also be used to produce various gases in the industry, such as acetylene or conversion of methane to higher hydrocarbon [
75]. Various atmospheric plasmas are also widely used in medicine (dentistry, dermatology, sterilization) and even to increase biocompatibility [
76]. Atmospheric plasma methods (corona, dielectric barrier discharge, plasma jet) are more attractive since on-line treatment is possible without the use of vacuum [
77,
78]. Moreover, these methods are cost effective, fast and easy to handle, and do not need expensive equipment, or cooling water [
79,
80]. Below are some atmospheric pressure plasma sources but the list is not exhaustive, only the most frequently used plasma sources are mentioned. By using specially designed electrodes, or changing the dielectric, individual plasmas can even be converted into each other (
Figure 1).
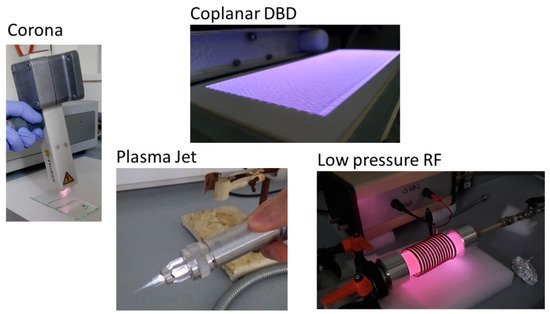
Figure 1. Some examples of atmospheric or low-pressure plasma from the author’s laboratory.
Electrical discharges are generated with relatively low current (μA to mA), usually in an air atmosphere. It typically develops between asymmetric (e.g., point-like) metal electrodes with the formation of an electron avalanche. It takes place under conditions in which a continuous arc discharge does not yet occur. Corona discharges have a small active volume and a fibrous structure. It is widely used for the surface treatment of polymeric materials. It is most commonly used to produce or remove surface electrostatic charges [
81].
It is also called silent discharge. It is not a new method, but it has only recently been used for surface treatment. Several types are known: volumetric (tubular or planar), and surface or coplanar DBD. It operates with alternating current (50–10 MHz), and in practice, it is a large number of micro-discharges (in pulse mode). It is suitable for forming relatively homogeneous plasmas with high energy density, low contact time and can be used together with continuous technologies. Two types of homogeneous atmospheric DBD plasmas are known: atmospheric pressure glow discharge (APGD) and Townsend DBD plasma (atmospheric pressure Townsend discharges, (APTS)) [
82]. Diffuse Coplanar Surface Barrier Discharge (DCSBD) is a special type of surface DBD and easy to apply in industry (
Figure 1) [
83].
Plasma jets are produced by generating non-thermal plasma in various gases or gas mixtures with a direct or alternating current with a wide frequency range (up to microwaves) and blowing it out of the source. The treatment is usually in the afterglow range. They are suitable for local treatment, e.g., to improve adhesion between two parts to be joined. Sometimes, with a suitable electrode design, a plasma jet can even be considered a DBD plasma [
84].
They are produced and sustained by high-frequency electromagnetic fields. RF and MW discharges are typically generated with a frequency of 1–100 MHz, and 0.3–10 GHz, respectively. Both are frequently used for etching, activation, and plasma polymerization. RF plasma can be capacitively or inductively coupled; in the latter case, the plasma is in no contact with the induction coil [
85].
1.2. Low-Pressure Plasma Treatment Applications
Low-pressure plasmas, which were invented earlier, are widely used in materials processing and surface treatment. They have some advantages over atmospheric plasmas, for example, a relatively high concentration of ions and radicals and uniform glow over a large gas volume, but they require vacuum during operation, which limits their use. Creating vacuum makes the whole process time and energy consuming, and requires expensive equipment. Therefore, some authors believe [
64] that low-pressure plasma systems are only appropriate for the preparation of value-added materials while atmospheric equipment is suitable for mass production.
2. Plasma Surface Interaction
Modification is usually limited to the outermost surface or some of its atomic layers, and bulk properties usually remain unchanged. The depth of treatment can be changed with the use of different types of plasma and different energy densities.
In the case of a polyethylene model material, in inductively coupled radiofrequency oxygen plasma a single monolayer is modified. Using microwave oxygen plasma, the modified layer is 5 nm thick, while in corona discharge air plasma it is thicker than 10 nm. The very slight difference can be attributed to the different reactivities of the excited particles (oxygen atom, singlet oxygen molecule, ions containing oxygen) in the plasmas [
86]. The depth of plasma treatment cannot be increased considerably by varying the parameters of the plasma, such as time, power, or gas. Therefore, reactions will not occur in the micrometer range.
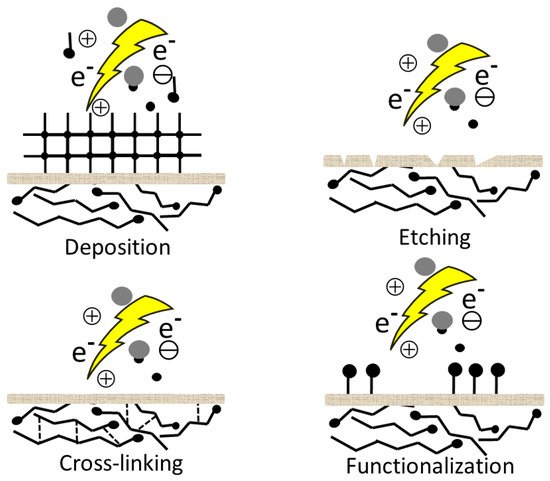
During plasma treatment, a number of processes takes place on the surfaces. These processes are highly dependent on the selected gas, the operating parameters and reactor design. Nevertheless, plasma–surface interactions are quite complex and not well understood [
87]. We present the current scientific knowledge with the details needed to understand this article. In general, the interaction between plasma and surface can be divided into two different types [
88]. One of them is when plasma is generated from non-polymerizing gases (e.g., N
2, O
2, Ar, air, and NH
3) and the major effect can be surface cleaning, etching, cross-linking, or formation of new functional groups and radicals simultaneously, because of the reactive particles in the plasma [
89,
90] (
Figure 2).
Figure 2. Plasma surface interaction.
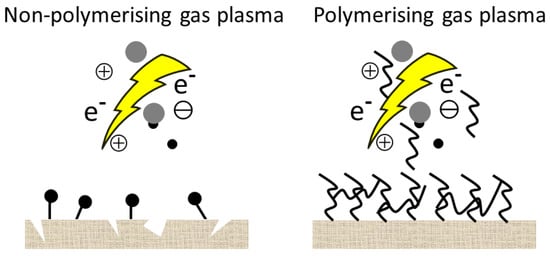
In this case, the surface becomes more hydrophilic mostly due to the newly formed functional groups or radicals. Since the hydrophilic surface itself is not desirable for the preservation of wood, in most cases, activation is followed by another reaction to tailor the properties of the surface. In the other case, the plasma gases are different polymerizable monomers or precursors (fluorocarbons, hydrocarbons, silicone-containing monomers, ethylene, or acetylene, etc.), which are converted by the plasma into reactive fragments. During recombination, a hydrophobic nanocoating will be deposited on the surface in a one-step reaction without any subsequent reaction. Sometimes a noble gas is used as a carrier gas during plasma polymerization. A schematic representation of the plasma treatment with two different types of gas is shown in Figure 3.
Figure 3. A schematic representation of the plasma treatment with two different types of gas.
2.1. Surface Cleaning
Plasma surface cleaning has already been used for a long time in industries such as metallurgy, and the production of microchips, semiconductors and medical plastics, etc. [
91]. During manufacture, organic contamination is always deposited on surfaces in layers of a few nanometers thick. It can hinder subsequent bonding or processing and can be easily removed by cold plasma. However, even if the aim is not to clean the surface, this cleaning process always occurs during plasma treatment
2.2. Functionalization
Low-pressure non-thermal discharges are usually applied for polymer surface functionalization. Air, O
2, N
2, NH
3, or CF
4 gases can generate functional groups containing O, N, H, and F (C=O, –COOH, –OH, –NH
2, etc.) on the surface [
92]. The resulting groups can produce hydrophobic or hydrophilic properties or promote the subsequent binding of molecules to establish the desired properties.
2.3. Radicals, Double Bonds and Cross-Linking
When the plasma is generated by noble gases, it produces active radicals on the surface by breaking the C–H bonds. The recombination of these radicals leads to the formation of double bonds or crosslinking of the molecular chains. The radicals and double bonds play an important role in anchoring molecules for grafting. Cross-linking is sometimes just an undesired side reaction, but there are cases where the goal is specifically to create a cross-linked surface [
93].
2.4. Etching
The objective of etching is to increase roughness by removing materials from the outmost layer generating volatile or low molecular weight by-products that desorb from the surface. It is a non-equilibrium process and often used in microelectronic industry [
94].
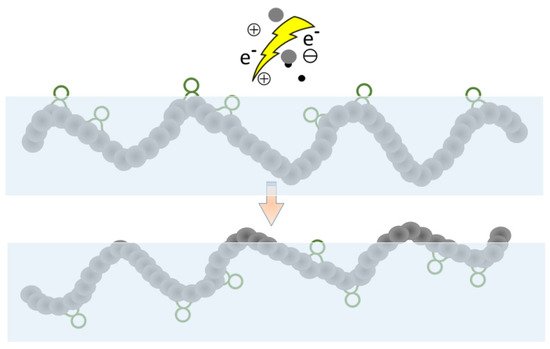
Most such processes are effective in the long term, but the formation of functional groups is only temporary and either a partial or complete hydrophobic recovery (
Figure 4) is usually observed [
95]. A possible explanation is the thermodynamically driven reorientation of the polar groups from the surface into the bulk. Therefore, plasma treatment is just the first step, and it is followed by a subsequent grafting reaction to obtain the desired surface property permanently. Usually, the plasma-activated surface can induce the grafting reactions, so this step does not require a solvent or a catalyst, either.
Figure 4. Hydrophobic recovery.
An additional advantage of plasma treatment is that not only esterification and etherification reactions take place. The active particles in the plasma can react oxygen and carbon atoms by the extraction of hydrogen, which results in oxidation, reduction, substitution, or establishment of an active radical, which is ready for a subsequent grafting [
96].
3. Characterization of the Effectiveness of Plasma Treatment
By systematically varying the plasma parameters, many researchers have tried to find the best settings to achieve the desired surface effect. Such parameters can be the choice of plasma gas, treatment time, or plasma power. With the choice of plasma gas, the incorporation of new functional groups can be planned in advance. For the incorporation of hydroxyl or carbonyl groups, oxygen or air plasma is required. De Farias [
97] found that oxygen plasma was far more effective than air plasma in terms of morphology and chemical changes with the same treatment time and power. They attributed this result to the stronger etching effect of oxygen plasma, as nitrogen is less reactive than oxygen in this environment. Treatment time is also a key parameter and not easy to determine. Some authors use a few seconds of treatment [
98], claiming that longer treatment times did not produce a more significant effect, or the sample was damaged seriously [
99], while others choose very long treatment times, sometimes up to 1 hour, without the destruction of the sample [
97]. It is very difficult to compare the different treatments, as in most cases, the plasmas are different even if produced in the same device. Plasma treatment does not only influence the chemistry of the materials but also results in surface roughening due to the etching effect of plasma. Both changes, morphological and chemical, play an important role in a further reaction on the surface, mostly grafting, and these changes can be examined by modern surface characterization methods. These methods apply photon (X-ray photoelectron spectroscopy (XPS)), electron (Auger electron spectroscopy (AES)) or ion beam (Ion Scattering Spectroscopy (ISS), Rutherford Backscattering Spectroscopy (RBS), Secondary Ion Mass Spectrometry (SIMS)) and can show the chemical composition of the surface, while surface imaging techniques (Scanning Electron Microscopy (SEM), Atomic Force Microscopy (AFM), Scanning Probe Microscopy (SPM), profilometry, as well as imaging XPS and AES) reveal morphological changes [
100].
3.1. XPS, Fourier Transform Infrared Spectroscopy (FTIR)
Researchers usually monitor changes in surface chemistry by XPS [
101] and FTIR. Plasma surface modification typically induces chemical changes in the outermost surface layer with a thickness of a few nanometers. XPS is considered the best technique for both the qualitative and quantitative chemical analysis of such thin layers because the penetration depth of XPS is roughly the same as that of plasma treatment [
102,
103,
104]. If a layered structure is developed during the treatment, the thickness of the layers can also be estimated from XPS data. In contrast, FTIR is less suitable for the analysis of a surface layer because FTIR provides information about the bulk material due to its large penetration depth (approximately a thousand times greater than that of XPS). This way, the signal from the surface is less intense, due to the limited number of functional groups. FTIR can give reliable results if bonds are formed during plasma treatment not present in the initial sample [
105].
3.2. SEM
Scanning electron microscopy is extensively used in the study of structural micro-morphological details on the surface of a sample [
106]. The high-resolution SEM images usually provide information about the topography, morphology, and composition of the surface. It is difficult to follow the elemental composition of wood by SEM as the components of wood (cellulose, lignin and hemicellulose) consist of the same elements (C, N, O). If the amount of one or more components changes, the elemental composition of the surface still remains the same.
3.3. Imaging XPS and Scanning AES
These techniques give structural information similar to SEM, although with limited lateral resolution (approx. 1 μm for XPS and 20 nm for AES). As extra advantage, elemental, in case of XPS also bonding state, composition data are supplied at the same time.
3.4. AFM
Morphological changes in the nanometer range can be monitored by atomic force microscopy [
107]. It is a very sensitive method for following surface changes, but over a far smaller magnification range than SEM [
108]. It is one of the best methods to monitor a polished plastic surface after plasma treatment, but examining wood is not easy. Owing to its composite nature, wood often exhibits macroscopic roughness, which is not in the AMF measurement range, so further increase in roughness can be measured with uncertainty.
This entry is adapted from the peer-reviewed paper 10.3390/coatings12040487