Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Breast cancer is the leading cause of cancer-related deaths in women worldwide. Long non-coding RNAs are newly described molecules that have extensive roles in breast cancer. Emerging reports have shown that there is a strong link between these RNAs and the hypoxic response of breast cancer cells, which may be an important factor for enhanced tumoral progression.
- long non-coding RNAs
- hypoxia
- breast cancer
- lncRNAs
1. Introduction
Breast cancer is the leading cause of cancer-related deaths in women worldwide. Its incidence has been increasing 0.5% per year since the last decade, mainly due to the decline in fertility rate and the increase in body weight among young women [1]. In the United States, even with earlier diagnosis and treatment improvements, the decline in mortality has stagnated in recent years, from a reported 3% to a 1% annually [2].
One of the most important mechanisms that drive breast cancer progression is hypoxia—a decrease in the microenvironment oxygen tension. Hypoxia in tumors results from the growth of aberrant new blood vessels developed during cancer progression, which cannot sustain an adequate blood supply. Tumor cells located more than 180 µm away from a vessel become anoxic and die by necrosis [3]. Cells in the immediate vicinity can survive in a chronic hypoxic condition by eliciting a strong cellular hypoxic response [4]. This response induces several local and distant physiological mechanisms, including a shift from aerobic to anaerobic cellular respiration, production of growth factors, pH regulation, proliferation, induction of distant production of erythropoietin from the kidney and local neo-angiogenesis.
2. Hypoxia Signaling Pathway
2.1. Canonical Hypoxia Signaling Pathway
Cells respond to hypoxia by a concerted physiological and sometimes pathological responses by activating the Hypoxia Signaling Pathway (HSP), which is centrally guided by a group of related proteins termed Hypoxia-Inducible-Factors (HIF). The HIF family is a conserved group of transcription factors that act as a heterodimer of alpha and beta subunits. In humans there are three alpha (HIF-1α, HIF-2α/EPAS and HIF-3α) and two beta paralogs (ARNT, ARNT2) [5]. In normoxic conditions, the canonical HIFα subunit, HIF-1α is bound to the von Hippel-Lindau (VHL) protein, allowing the activation of the ubiquitin ligase system, which renders it susceptible to its degradation by the proteasomal degradation complex (Figure 1). To bind VHL, HIF-1α proline residues need to be hydroxylated, a process that depends on several hydroxylases, including α-ketoglutarate-dependent dioxygenases and the prolyl hydroxylases (PHD). In addition, hydroxylation of an asparagine in the C-terminal transactivation domain by the asparaginyl hydroxylase (factor-inhibiting HIF (FIH)) prevents its interaction with the p300 coactivator and thus HIF transcriptional activity [6][7]. An oxygen level decrease inhibits the PHD and FIH, leading to a reduction in hydroxylation and thus, HIF-1α stabilization. Higher levels of this unit allow it to dimerize with the HIF-1β subunit, which induces their translocation to the nucleus. There, the dimer recruits additional co-activators such as CREB and p300 and acts as a transcription factor that binds to E-box-like hypoxia response elements (HREs) (5′-RCGTG-3′) in a diverse array of hypoxia-inducible promoters in at least a couple hundred genes, as described by integrative approaches [8]. The products of these genes not only regulate various biological processes, including cellular metabolism, growth, apoptosis, and migration, but also include several oncogenes and tumor suppressor genes. Since these same processes and genes are involved during carcinogenesis, it is not surprising that hypoxia is a key tumoral micro-ambient factor involved in cancer progression.
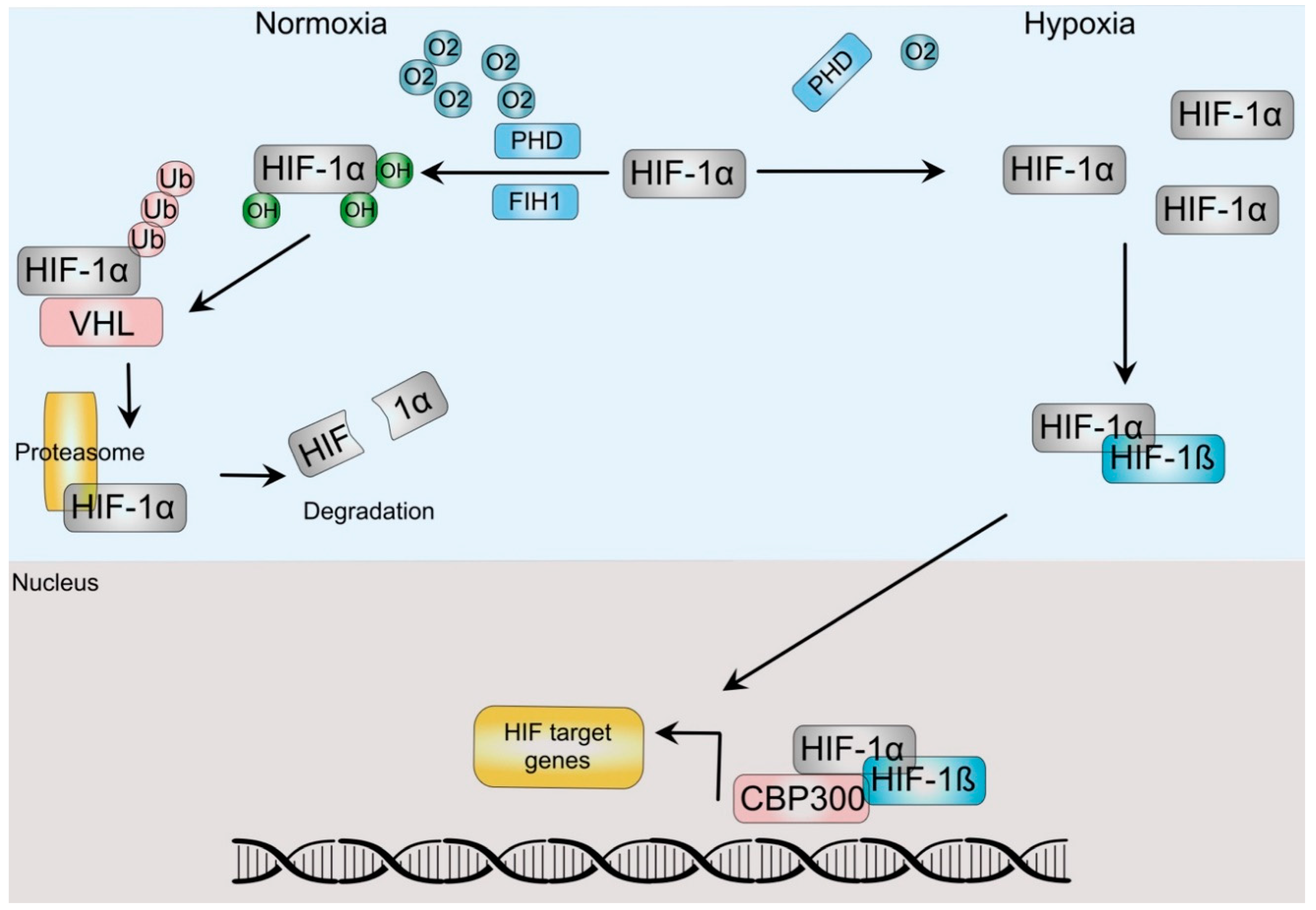
Figure 1. HIF-dependent hypoxia signaling pathway. In normoxia, prolyl-hydrolyses (PHD) and Factor-inhibiting hypoxia-inducible factor (FIH) hydroxylate HIF-α. Hydroxylation from the latter impairs HIF transactivation of target genes, whereas the former allows dimerization with the Von Hippel Lindau (VHL) protein, which directs HIF to ubiquitination and degradation by the proteasome. Hypoxic conditions stabilize HIF-α after PHD and FIH inactivation, allowing dimerization with HIF-ß subunits, translocation to the nucleus and association with coactivators, such as p300/CBP to regulate gene expression.
2.2. The Hypoxia Signaling Pathway and Breast Cancer
Most solid tumors have a hypoxic environment, which correlates with poor clinical outcomes. Early reports by Hockey et al. demonstrated that low oxygen in tumors was associated with increased metastasis and lower survival in patients with breast cancer [9]. Indeed, it has been estimated that 40% of all breast tumors and 50% of locally advanced breast cancers have hypoxic regions at the time of diagnosis [10], which add to the role of hypoxia during the early tumor progression. In common with other tumors, breast cancer tissues present higher levels of HIF-1α and hypoxia, which correlates with poor prognosis, including early relapse and metastatic disease [11]. As expected by the oxygen diffusion ability, breast precursor lesions such as ductal carcinoma in situ (DCIS) and early stage breast cancer already present HIF-1α overexpression [12].
The HSP is key to two main cancer progression processes: angiogenesis and metabolic reprogramming. As stated before, oxygen diffusion is limited to 180 µm, so tumoral growth is restricted to masses not larger than 1–2 mm before becoming hypoxic [13]. After reaching that volume, the HSP induces a complex stress response aimed mainly at supporting neo-angiogenesis and metabolic reprogramming [14]. This process is not straight-forward, since most of the new vessels are disorganized and leaky, which further increases hypoxic areas. Neo-angiogenesis relies heavily in the HSP, mediated by classical angiogenic inducers such as VEGF and Angiopoietin-like factors and angiogenesis receptors (e.g., VEGFR, ANGPTR) and microenvironment matrix elements that act not only in the tumor cells themselves, but also in the tumor endothelial cells [15]. In breast cancer, several reports [16][17][18] have shown that a complex interplay between tumor and stromal cells create a pro-angiogenic environment in which the HSP mediated by HIF members is the key regulator, as shown by loss-of-function experiments [19].
The second important oncogenic process regulated by the HSP is metabolic reprogramming, which includes carbohydrates, amino acids, and lipids. The main example is the modulation of the cellular energetic metabolism by hypoxia. In this case, HIF-1α induces a shift from mitochondrial respiration to glycolytic-dependent metabolism. This is achieved through up-regulation of glycolytic enzymes and pyruvate redistribution toward lactate production by several coupled mechanisms [20]. In parallel, hypoxia induces a concurrent increase in production and secretion of lactate, which acidifies the extracellular milieu [21]. Increases in glycogen synthesis and glucose uptake also accompanied this change, which add to a chemoresistance phenotype of breast cancer cells [22]. Accumulation of extracellular lactate and consequent acidification contribute to an important immunosuppressive microenvironment in breast tumors [23]. The principal actors in these changes are the HIF-responsive carbonic anhydrase 9 and monocarboxylate transporters (MCT) 1 and 4 [23][24]. The former catalyzes the conversion of CO2 and water to HCO3- and H+, whereas the latter mediates the lactate and H+ efflux from breast cancer cells [21][25]. Since most of the pyruvate in cancer cells is redirected away from the Krebs cycle, hypoxic cells require additional sources of Krebs cycle intermediates, such as cysteine and glutamine. This is achieved by an up regulation of several amino acid importers, such as SNAT2, SLC1A5, ASCT2, SLC7A11 and SLC7A5, all of which are HIF-responsive genes [26] and by an increase in the enzyme glutaminase, which converts glutamine to glutamate [27]. Cancer cells require fatty acids and lipids to support key ongoing oncogenic processes such as metabolism, signaling, intracellular oxidative adaptation and growth [28]. HIF proteins represses fatty acid oxidation and up regulates their synthesis by transactivating the genes of several enzymes involved in this process, such as the fatty acid synthase, lipin 1 and acetyl-CoA carboxylase (ACC). Simultaneously, the HSP is involved in an important increase in fatty acid uptake by up regulating fatty acid-binding proteins [29].
More recently, several authors have found that the HSP can modulate not only transient transcriptional responses, but also epigenetic programs. This is accomplished by changing the methylation status of both DNA and nuclear histones [30]. Induction of histone lysine demethylases (KDM) by the HSP is key to this process, as they stabilize HIF-1α complexes to initiate transcription of its key target genes. In addition, several KDM are members of the 2-oxoglutarate-dependent dioxygenase family (KDM3A, KDM2B, KDM4B, KDM5B, KDM6B and KDM4C), so they depend on oxygen and 2-oxoglutarate to present their enzymatic activity [30]. These enzymes act also as signal amplifiers and transcriptional facilitators for the expression of genes downstream of HIF signaling [31] and also mediate chromatin rearrangements to facilitate this [32]. Similarly, hypoxia reduces the activity of the TET demethylases, inducing DNA hyper-methylation [33]. Pediatric ependymomas underline the importance of hypoxia-mediated epigenetic reprogramming in cancer. As many of other pediatric tumors, these cancers have a very low number of recurrent mutations, so an epigenetic origin has been suggested. Ependymomas arise from putative stem cells embedded in a hypoxic compartment, in which the lack of oxygen establishes a gene regulatory program that depends on it [34][35]. These results show the HSP ability to regulate the epigenetic machinery to provide a stable response to hypoxia.
Several research groups have shown that a large part of what previously was considered “junk” DNA is actually transcribed [36] and at least part of it has important cellular functions [37][38]. Among the important transcribed DNA regions, non-coding RNAs genes have proven to be an enormous source of previously unknown regulatory elements that take part in physiological and pathological states. Among the latter, cancer stands out, perhaps due to the complex and profound genomic rearrangements that characterize it [39]. Non-coding RNAs can be arbitrarily separated into two groups, small non-coding RNAs (sncRNAs) and long non-coding RNAs (lncRNAs), according to their length. Long non-coding RNAs (lncRNAs) are transcripts longer than 200 base pairs transcribed from intergenic or even genic regions. They are classified by its molecular function as decoy lncRNAs, when they sequester proteins, guide lncRNAs, which can recruit chromatin modifiers, scaffolding lncRNAs that act as protein adaptors and sponges, which act as competing endogenous molecules (ceRNAs) that prevent microRNAs to interact with mRNAs by being “sponges” and enhancer lncRNAs that stabilize chromosome loops [40]. These molecular activities allow them to take part in almost all cellular processes explored to date, as they converge into transcriptional and postranscriptional mechanisms, epigenetic modulation and even signal transduction participation. As expected by these facts, there are many reports showing deregulation of several lncRNAs in a long list of tumors [41]. Changes in lncRNAs expression have been also associated with clinical characteristics, prompting several authors to propose the use of them as diagnostic or prognostic tools [42].
In breast cancer, lncRNAs have been extensively studied, but their role seems to be more complex that initially thought. These molecules participate in breast cancer cell proliferation [43][44], invasion [45], migration [44][45][46], apoptosis [47], epithelial-mesenchymal transition (EMT) [48], stem phenotype [49] and response to chemotherapeutic drugs [40][50] and most of the described cancer hallmarks [51]. Most of the studies have focused on the cell-autonomous effects of these RNAs, leaving the role of non-cell autonomous signaling provided by the Tumor Microenvironment (TME) unexplored. Since there is a remarkable intra- and inter-tumoral heterogeneity in breast cancer, more efforts are needed to study the role of the surrounding TME in gene expression regulation. In addition, recent reports have also shown that these non-coding RNAs can also take part in emerging cancer hallmarks such as phenotypic plasticity and non-mutational epigenetic reprogramming (NMER) [52]. TME is thus a key component required for full cancer progression, as it not only provides an adequate niche for tumor development, but also interacts with cancer cells in a bidirectional manner [53].
3. Hypoxia-Associated Long Non-Coding RNAs as Regulators of Breast Cancer
As stated previously, lncRNAs are critically involved in a bidirectional signaling circuit between TME and cancer cells. Two TME-related factors are key to drive cancer progression by providing important evolutionary forces involved in it: hypoxia and acidosis.
Recently, Lin et al. reported the discovery of hypoxia-regulated lncRNAs in breast cancer cells [54]. These authors used a RNASeq approach in MCF-7 cells exposed to normoxia, hypoxia and re-oxygenation conditions and found 472 lncRNAs that were differentially regulated during hypoxic conditions, validating three of them: lnc-CPN2-1, lnc-C11orf35-2 and lnc-NDRG1-1. Although not comprehensive since it was produced using only one cell line, this report showed that the number of hypoxia-responsive lncRNAs could be counted into the hundreds. A second article published in 2021 used two breast cancer cell lines to perform a similar RNASeq-based analysis [55]. Here, 104 and 282 regulated lncRNAs were found in each cell line, and only 43 were shared between them. Twenty-six RNAs were validated, but interestingly, 17 were not. Results from this study could help to provide more accurate network analyses and prognostic markers. For example, Gong, P. J. et al. recently used a panel of 13 hypoxia-related lncRNAs to generate a classification or “clusters” of breast cancer patients based on this signature [56]. The cluster with an unregulated hypoxic signature presented a differential immune infiltration profile, with lower CD8+ and CD4+ T but higher nTreg and iTreg cells number.
Most of the reports that have analyzed the role of lncRNA in the HSP have focused on RNAs that respond to hypoxia, although there are also several reports that have shown a direct role of lncRNAs that regulate the HSP and are even able to establish a positive signaling feedback to amplify the HSP [57][58]. Most of these reports analyzed lncRNAs that were previously associated with hypoxia in other tissues, so a more comprehensive discovery effort, as stated, should help to direct the efforts to the more relevant RNAs.
The cellular processes modulated by hypoxic-associated lncRNAs are diverse, but most authors focused on proliferation, migration and invasion, epithelial-mesenchymal transition (EMT) and glycolysis, as these are probably the most studied hallmarks of cancer [51]. Additional processes, such as apoptosis, stemness, and angiogenesis, were less studied, whereas recently described cancer hallmarks, such as non-mutational epigenetic reprogramming or senescence, have not been explored at all.
4. Hypoxia-Associated lncRNAs as Breast Cancer Clinical Molecular Markers
As expected by the role of hypoxia in cancer, several research groups have explored the potential use of hypoxia-related lncRNAs as molecular markers. In 2017, Liu et al., created a 3-lncRNA signature that could classify breast tumors as triple-negative or non-triple negative tumors based on plasma obtained from patients [59]. Gong et al. used a 13-gene signature that included four lncRNAs for predicting poor prognosis of breast cancer patients using a network approach that also uncovered immunological differences [56]. A more recent work using a four-lncRNA signature had prognostic power for overall survival in patients with breast cancer when used to stratify patients in low- and high-risk groups [60]. Gu et al. recently proposed a more extensive signature with 12 lncRNAs [61]. This signature was used to predict the survival outcome, classifying breast cancer patients in high and low-risk groups. Patients in the high-risk group had shorter median overall and disease-free survival and lower chemosensitivity compared with those in the low-risk group. More importantly, the score provided by these authors was an independent prognostic factor. Finally, there are additional works that have explored the use of single lncRNAs as markers, such as the report from Wang et al. in 2019, which found that HIF1A-AS2 expression had prognostic value for lymph node and distant metastasis, unfavorable histological grade and shorter overall survival [62].
This entry is adapted from the peer-reviewed paper 10.3390/cells11101679
References
- Pfeiffer, R.M.; Webb-Vargas, Y.; Wheeler, W.; Gail, M.H. Proportion of U.S. Trends in Breast Cancer Incidence Attributable to Long-term Changes in Risk Factor Distributions. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1214–1222.
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33.
- Thomlinson, R.H.; Gray, L.H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer 1955, 9, 539–549.
- Ivan, M.; Fishel, M.L.; Tudoran, O.M.; Pollok, K.E.; Wu, X.; Smith, P.J. Hypoxia signaling: Challenges and opportunities for cancer therapy. Semin. Cancer Biol. 2021.
- Graham, A.M.; Presnell, J.S. Hypoxia Inducible Factor (HIF) transcription factor family expansion, diversification, divergence and selection in eukaryotes. PLoS ONE 2017, 12, e0179545.
- Myllyharju, J. Prolyl 4-hydroxylases, master regulators of the hypoxia response. Acta Physiol. 2013, 208, 148–165.
- Yu, M.; Lun, J.; Zhang, H.; Zhu, L.; Zhang, G.; Fang, J. The non-canonical functions of HIF prolyl hydroxylases and their dual roles in cancer. Int. J. Biochem. Cell Biol. 2021, 135, 105982.
- Ortiz-Barahona, A.; Villar, D.; Pescador, N.; Amigo, J.; del Peso, L. Genome-wide identification of hypoxia-inducible factor binding sites and target genes by a probabilistic model integrating transcription-profiling data and in silico binding site prediction. Nucleic Acids Res. 2010, 38, 2332–2345.
- Hockel, M.; Vaupel, P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 2001, 93, 266–276.
- Vaupel, P.; Briest, S.; Hockel, M. Hypoxia in breast cancer: Pathogenesis, characterization and biological/therapeutic implications. Wien. Med. Wochenschr. 2002, 152, 334–342.
- Dales, J.P.; Garcia, S.; Meunier-Carpentier, S.; Andrac-Meyer, L.; Haddad, O.; Lavaut, M.N.; Allasia, C.; Bonnier, P.; Charpin, C. Overexpression of hypoxia-inducible factor HIF-1alpha predicts early relapse in breast cancer: Retrospective study in a series of 745 patients. Int. J. Cancer 2005, 116, 734–739.
- Bos, R.; Zhong, H.; Hanrahan, C.F.; Mommers, E.C.; Semenza, G.L.; Pinedo, H.M.; Abeloff, M.D.; Simons, J.W.; van Diest, P.J.; van der Wall, E. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J. Natl. Cancer Inst. 2001, 93, 309–314.
- Munoz, L.; Espinosa, M.; Quintanar-Jurado, V.; Hidalgo, A.; Melendez-Zajgla, J.; Maldonado, V. Paradoxial changes in the expression of estrogen receptor alpha in breast cancer multicellular spheroids. Tissue Cell 2010, 42, 334–337.
- Munoz-Galindo, L.; Melendez-Zajgla, J.; Pacheco-Fernandez, T.; Rodriguez-Sosa, M.; Mandujano-Tinoco, E.A.; Vazquez-Santillan, K.; Castro-Oropeza, R.; Lizarraga, F.; Sanchez-Lopez, J.M.; Maldonado, V. Changes in the transcriptome profile of breast cancer cells grown as spheroids. Biochem. Biophys. Res. Commun. 2019, 516, 1258–1264.
- Hashimoto, T.; Shibasaki, F. Hypoxia-inducible factor as an angiogenic master switch. Front. Pediatr. 2015, 3, 33.
- Palazon, A.; Tyrakis, P.A.; Macias, D.; Velica, P.; Rundqvist, H.; Fitzpatrick, S.; Vojnovic, N.; Phan, A.T.; Loman, N.; Hedenfalk, I.; et al. An HIF-1alpha/VEGF-A Axis in Cytotoxic T Cells Regulates Tumor Progression. Cancer Cell 2017, 32, 669–683.e5.
- Incio, J.; Ligibel, J.A.; McManus, D.T.; Suboj, P.; Jung, K.; Kawaguchi, K.; Pinter, M.; Babykutty, S.; Chin, S.M.; Vardam, T.D.; et al. Obesity promotes resistance to anti-VEGF therapy in breast cancer by up-regulating IL-6 and potentially FGF-2. Sci. Transl. Med. 2018, 10, eaag0945.
- Semenza, G.L. The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochim. Biophys. Acta 2016, 1863, 382–391.
- Tang, N.; Wang, L.; Esko, J.; Giordano, F.J.; Huang, Y.; Gerber, H.P.; Ferrara, N.; Johnson, R.S. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell 2004, 6, 485–495.
- Semenza, G.L. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J. Clin. Investig. 2013, 123, 3664–3671.
- Jamali, S.; Klier, M.; Ames, S.; Barros, L.F.; McKenna, R.; Deitmer, J.W.; Becker, H.M. Hypoxia-induced carbonic anhydrase IX facilitates lactate flux in human breast cancer cells by non-catalytic function. Sci. Rep. 2015, 5, 13605.
- Altemus, M.A.; Goo, L.E.; Little, A.C.; Yates, J.A.; Cheriyan, H.G.; Wu, Z.F.; Merajver, S.D. Breast cancers utilize hypoxic glycogen stores via PYGB, the brain isoform of glycogen phosphorylase, to promote metastatic phenotypes. PLoS ONE 2019, 14, e0220973.
- Boedtkjer, E. Na+,HCO3- cotransporter NBCn1 accelerates breast carcinogenesis. Cancer Metastasis Rev. 2019, 38, 165–178.
- Wykoff, C.C.; Beasley, N.J.; Watson, P.H.; Turner, K.J.; Pastorek, J.; Sibtain, A.; Wilson, G.D.; Turley, H.; Talks, K.L.; Maxwell, P.H.; et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000, 60, 7075–7083.
- Ames, S.; Andring, J.T.; McKenna, R.; Becker, H.M. CAIX forms a transport metabolon with monocarboxylate transporters in human breast cancer cells. Oncogene 2020, 39, 1710–1723.
- de Heer, E.C.; Jalving, M.; Harris, A.L. HIFs, angiogenesis, and metabolism: Elusive enemies in breast cancer. J. Clin. Investig. 2020, 130, 5074–5087.
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634.
- Butler, L.M.; Perone, Y.; Dehairs, J.; Lupien, L.E.; de Laat, V.; Talebi, A.; Loda, M.; Kinlaw, W.B.; Swinnen, J.V. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 2020, 159, 245–293.
- Rohrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749.
- Choudhry, H.; Harris, A.L. Advances in Hypoxia-Inducible Factor Biology. Cell Metab. 2018, 27, 281–298.
- Choudhry, H.; Schodel, J.; Oikonomopoulos, S.; Camps, C.; Grampp, S.; Harris, A.L.; Ratcliffe, P.J.; Ragoussis, J.; Mole, D.R. Extensive regulation of the non-coding transcriptome by hypoxia: Role of HIF in releasing paused RNApol2. EMBO Rep. 2014, 15, 70–76.
- Platt, J.L.; Salama, R.; Smythies, J.; Choudhry, H.; Davies, J.O.; Hughes, J.R.; Ratcliffe, P.J.; Mole, D.R. Capture-C reveals preformed chromatin interactions between HIF-binding sites and distant promoters. EMBO Rep. 2016, 17, 1410–1421.
- Thienpont, B.; Steinbacher, J.; Zhao, H.; D’Anna, F.; Kuchnio, A.; Ploumakis, A.; Ghesquiere, B.; Van Dyck, L.; Boeckx, B.; Schoonjans, L.; et al. Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature 2016, 537, 63–68.
- Lin, G.L.; Monje, M. Understanding the Deadly Silence of Posterior Fossa A Ependymoma. Mol. Cell 2020, 78, 999–1001.
- Michealraj, K.A.; Kumar, S.A.; Kim, L.J.Y.; Cavalli, F.M.G.; Przelicki, D.; Wojcik, J.B.; Delaidelli, A.; Bajic, A.; Saulnier, O.; MacLeod, G.; et al. Metabolic Regulation of the Epigenome Drives Lethal Infantile Ependymoma. Cell 2020, 181, 1329–1345.e24.
- Pennisi, E. Genomics. ENCODE project writes eulogy for junk DNA. Science 2012, 337, 1159–1161.
- Ge, S.X. Exploratory bioinformatics investigation reveals importance of “junk” DNA in early embryo development. BMC Genom. 2017, 18, 200.
- Bernardi, G. The “Genomic Code”: DNA Pervasively Moulds Chromatin Structures Leaving no Room for “Junk”. Life 2021, 11, 342.
- Ling, H.; Vincent, K.; Pichler, M.; Fodde, R.; Berindan-Neagoe, I.; Slack, F.J.; Calin, G.A. Junk DNA and the long non-coding RNA twist in cancer genetics. Oncogene 2015, 34, 5003–5011.
- Melendez-Zajgla, J.; Maldonado, V. The Role of lncRNAs in the Stem Phenotype of Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2021, 22, 6374.
- Jiang, M.C.; Ni, J.J.; Cui, W.Y.; Wang, B.Y.; Zhuo, W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am. J. Cancer Res. 2019, 9, 1354–1366.
- Qian, Y.; Shi, L.; Luo, Z. Long Non-coding RNAs in Cancer: Implications for Diagnosis, Prognosis, and Therapy. Front. Med. 2020, 7, 612393.
- Xiping, Z.; Bo, C.; Shifeng, Y.; Feijiang, Y.; Hongjian, Y.; Qihui, C.; Binbin, T. Roles of MALAT1 in development and migration of triple negative and Her-2 positive breast cancer. Oncotarget 2018, 9, 2255–2267.
- Fernandez-Rojas, M.A.; Melendez-Zajgla, J.; Lagunas, V.M. lincRNA-RP11400K9.4 Regulates Cell Survival and Migration of Breast Cancer Cells. Cancer Genom. Proteom. 2020, 17, 769–779.
- Wang, J.; Chen, X.; Hu, H.; Yao, M.; Song, Y.; Yang, A.; Xu, X.; Zhang, N.; Gao, J.; Liu, B. PCAT-1 facilitates breast cancer progression via binding to RACK1 and enhancing oxygen-independent stability of HIF-1α. Mol. Ther. Nucleic Acids 2021, 24, 310–324.
- Shi, G.; Cheng, Y.; Zhang, Y.; Guo, R.; Li, S.; Hong, X. Long non-coding RNA LINC00511/miR-150/MMP13 axis promotes breast cancer proliferation, migration and invasion. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 165957.
- Wang, X.; Chen, T.; Zhang, Y.; Zhang, N.; Li, C.; Li, Y.; Liu, Y.; Zhang, H.; Zhao, W.; Chen, B.; et al. Long noncoding RNA Linc00339 promotes triple-negative breast cancer progression through miR-377-3p/HOXC6 signaling pathway. J. Cell. Physiol. 2019, 234, 13303–13317.
- Wang, Y.; Dong, T.; Wang, P.; Li, S.; Wu, G.; Zhou, J.; Wang, Z. LINC00922 regulates epithelial-mesenchymal transition, invasive and migratory capacities in breast cancer through promoting NKD2 methylation. Cell. Signal. 2021, 77, 109808.
- Lu, G.; Li, Y.; Ma, Y.; Lu, J.; Chen, Y.; Jiang, Q.; Qin, Q.; Zhao, L.; Huang, Q.; Luo, Z.; et al. Long noncoding RNA LINC00511 contributes to breast cancer tumourigenesis and stemness by inducing the miR-185-3p/E2F1/Nanog axis. J. Exp. Clin. Cancer Res. 2018, 37, 289.
- Du, T.; Shi, Y.; Xu, S.; Wan, X.; Sun, H.; Liu, B. Long Non-Coding RNAs in Drug Resistance of Breast Cancer. Onco Targets Ther. 2020, 13, 7075–7087.
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46.
- Gagliano, T.; Brancolini, C. Epigenetic Mechanisms beyond Tumour-Stroma Crosstalk. Cancers 2021, 13, 914.
- Li, J.J.; Tsang, J.Y.; Tse, G.M. Tumor Microenvironment in Breast Cancer-Updates on Therapeutic Implications and Pathologic Assessment. Cancers 2021, 13, 4233.
- Lin, H.C.; Yeh, C.C.; Chao, L.Y.; Tsai, M.H.; Chen, H.H.; Chuang, E.Y.; Lai, L.C. The hypoxia-responsive lncRNA NDRG-OT1 promotes NDRG1 degradation via ubiquitin-mediated proteolysis in breast cancer cells. Oncotarget 2018, 9, 10470–10482.
- Chen, L.; Bao, L.; Niu, Y.; Wang, J.E.; Kumar, A.; Xing, C.; Wang, Y.; Luo, W. LncIHAT Is Induced by Hypoxia-Inducible Factor 1 and Promotes Breast Cancer Progression. Mol. Cancer Res. 2021, 19, 678–687.
- Gong, P.J.; Shao, Y.C.; Huang, S.R.; Zeng, Y.F.; Yuan, X.N.; Xu, J.J.; Yin, W.N.; Wei, L.; Zhang, J.W. Hypoxia-Associated Prognostic Markers and Competing Endogenous RNA Co-Expression Networks in Breast Cancer. Front. Oncol. 2020, 10, 579868.
- Liu, X.; Qiao, K.; Zhu, K.; Li, X.; Zhao, C.; Li, J.; Feng, D.; Fang, Y.; Wang, P.; Qian, C.; et al. Long Noncoding RNA HCG18 Promotes Malignant Phenotypes of Breast Cancer Cells via the HCG18/miR-103a-3p/UBE2O/mTORC1/HIF-1α-Positive Feedback Loop. Front. Cell Dev. Biol. 2021, 9, 675082.
- Zheng, F.; Chen, J.; Zhang, X.; Wang, Z.; Chen, J.; Lin, X.; Huang, H.; Fu, W.; Liang, J.; Wu, W.; et al. The HIF-1α antisense long non-coding RNA drives a positive feedback loop of HIF-1α mediated transactivation and glycolysis. Nat. Commun. 2021, 12, 1341.
- Liu, M.; Xing, L.Q.; Liu, Y.J. A three-long noncoding RNA signature as a diagnostic biomarker for differentiating between triple-negative and non-triple-negative breast cancers. Medicine 2017, 96, e6222.
- Zhao, Y.; Liu, L.; Zhao, J.; Du, X.; Yu, Q.; Wu, J.; Wang, B.; Ou, R. Construction and Verification of a Hypoxia-Related 4-lncRNA Model for Prediction of Breast Cancer. Int. J. Gen. Med. 2021, 14, 4605–4617.
- Gu, P.; Zhang, L.; Wang, R.; Ding, W.; Wang, W.; Liu, Y.; Wang, W.; Li, Z.; Yan, B.; Sun, X. Development and Validation of a Novel Hypoxia-Related Long Noncoding RNA Model With Regard to Prognosis and Immune Features in Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 796729.
- Wang, Y.; Zhang, G.; Han, J. HIF1A-AS2 predicts poor prognosis and regulates cell migration and invasion in triple-negative breast cancer. J. Cell. Biochem. 2019, 120, 10513–10518.
This entry is offline, you can click here to edit this entry!
