As essential components of people's connective tissues, elastic fibres give tissues such as major blood vessels, skin and the lungs their elasticity. In humans, the fibrillin family is composed of three highly conserved proteins, fibrillin-1, -2 and -3, all of which are engaged in the formation of microfibrils. Fibrillin-2 and -3 are mainly expressed in fetal tissues, while fibrillin-1 is continuously expressed throughout adulthood in tissues such as the heart, aorta, lung, nervous system and skin. Mutations in the FBN1 gene, which encodes fibrillin-1, are associated with MFS, isolated autosomal dominant ectopia lentis 1, mitral valve-aorta-skeleton-skin (MASS) syndrome, Weill–Marchesani syndrome (WMS), stiff skin syndrome, acromicric and geleophysic dysplasias and Marfanoid-progeroid-lipodystrophy syndrome.
- elastic fibre proteins
- wound healing
- fibrillin-1
- LTBP4
- tropoelastin
- TGFβ signalling
- integrin
1. Introduction
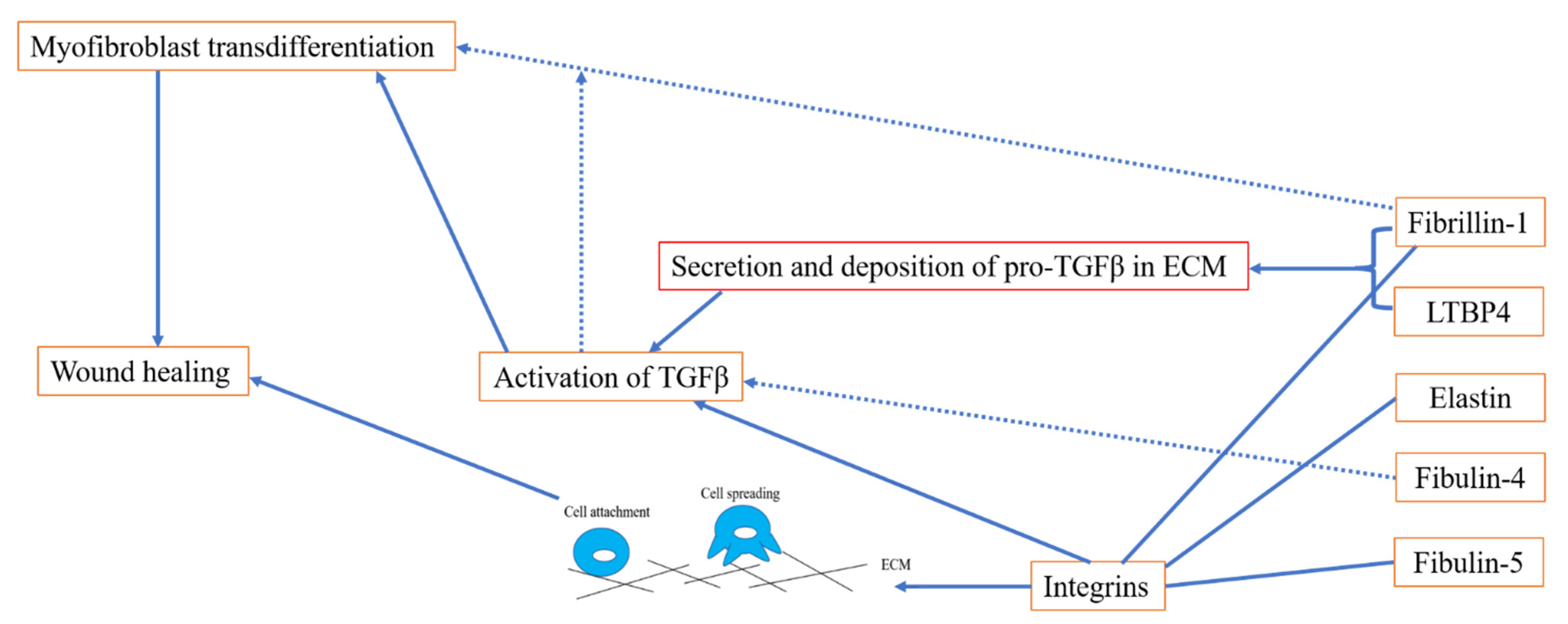
Elastic fibres endow tissues and organs with elasticity and extendibility in response to mechanical forces. Aberrant formation and destruction of elastic fibres leads to many diseases, such as Marfan syndrome (MFS) [1], cutis laxa and aneurysms [2]. Elastic fibres are formed predominantly from elastin and fibrillin microfibrils [3]. Elastic fibre proteins guide and facilitate elastogenesis, where tropoelastin globules are deposited on a fibrillin microfibril scaffold, a process which is facilitated by fibulin-4 and -5 and latent TGFβ-binding protein (LTBP)-4. In addition to elastogenesis, elastic fibre proteins have been implicated in wound healing: for instance, in keloid disease and hypertrophic scarring, disorganised and reduced elastin and fibrillin has been observed [4][5]. Furthermore, elastic fibre proteins are important players in regulating TGFβ signalling [6] and integrin-mediated cell attachment and spreading, which can further contribute to wound healing.
2. The Role of Elastic Fibre Proteins in Wound Repair
2.1. Elastic Fibre Proteins and TGFβ Signalling
2.2. Role of Elastic Fibre Proteins Supporting Integrin-Mediated Cell Adhesion
3. Perspectives
This entry is adapted from the peer-reviewed paper 10.3390/ijms23084087
References
- Ramirez, F.; Caescu, C.; Wondimu, E.; Galatioto, J. Marfan syndrome; A connective tissue disease at the crossroads of mechanotransduction, TGFbeta signaling and cell stemness. Matrix. Biol. 2018, 71–72, 82–89.
- Halabi, C.M.; Kozel, B.A. Vascular elastic fiber heterogeneity in health and disease. Curr. Opin. Hematol. 2020, 27, 190–196.
- Thomson, J.; Singh, M.; Eckersley, A.; Cain, S.A.; Sherratt, M.J.; Baldock, C. Fibrillin microfibrils and elastic fibre proteins: Functional interactions and extracellular regulation of growth factors. Semin. Cell Dev. Biol. 2019, 89, 109–117.
- Ghazawi, F.M.; Zargham, R.; Gilardino, M.S.; Sasseville, D.; Jafarian, F. Insights into the pathophysiology of hypertrophic scars and keloids: How do they differ? Adv. Skin Wound Care 2018, 31, 582–595.
- Jumper, N.; Paus, R.; Bayat, A. Functional histopathology of keloid disease. Histol. Histopathol. 2015, 30, 11033–11057.
- Godwin, A.; Singh, M.; Lockhart-Cairns, M.P.; Alanazi, Y.F.; Cain, S.A.; Baldock, C. The role of fibrillin and microfibril binding proteins in elastin and elastic fibre assembly. Matrix. Biol. 2019, 84, 17–30.
- Handa, K.; Abe, S.; Suresh, V.V.; Fujieda, Y.; Ishikawa, M.; Orimoto, A.; Kobayashi, Y.; Yamada, S.; Yamaba, S.; Murakami, S.; et al. Fibrillin-1 insufficiency alters periodontal wound healing failure in a mouse model of Marfan syndrome. Arch. Oral. Biol. 2018, 90, 53–60.
- Yoshiba, N.; Yoshiba, K.; Ohkura, N.; Takei, E.; Edanami, N.; Oda, Y.; Hosoya, A.; Nakamura, H.; Okiji, T. Correlation between Fibrillin-1 Degradation and mRNA Downregulation and Myofibroblast Differentiation in Cultured Human Dental Pulp Tissue. J. Histochem. Cytochem. 2015, 63, 438–448.
- Lee, M.J.; Roy, N.K.; Mogford, J.E.; Schiemann, W.P.; Mustoe, T.A. Fibulin-5 promotes wound healing in vivo. J. Am. Coll. Surg. 2004, 199, 403–410.
- Almine, J.F.; Wise, S.G.; Hiob, M.; Singh, N.K.; Tiwari, K.K.; Vali, S.; Abbasi, T.; Weiss, A.S. Elastin sequences trigger transient proinflammatory responses by human dermal fibroblasts. FASEB J. 2013, 27, 3455–3465.
- Margadant, C.; Sonnenberg, A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010, 11, 97–105.
- Zilberberg, L.; Todorovic, V.; Dabovic, B.; Horiguchi, M.; Couroussé, T.; Sakai, L.Y.; Rifkin, D.B. Specificity of latent TGF-beta binding protein (LTBP) incorporation into matrix: Role of fibrillins and fibronectin. J. Cell. Physiol. 2012, 227, 3828–3836.
- Miyazono, K.; Olofsson, A.; Colosetti, P.; Heldin, C.H. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J. 1991, 10, 1091–1101.
- Annes, J.P.; Chen, Y.; Munger, J.S.; Rifkin, D.B. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J. Cell. Biol. 2004, 165, 723–734.
- Lamar, K.M.; Miller, T.; Dellefave-Castillo, L.; McNally, E.M. Genotype-Specific Interaction of Latent TGFbeta Binding Protein 4 with TGFbeta. PLoS ONE 2016, 11, e0150358.
- Su, C.T.; Huang, J.W.; Chiang, C.K.; Lawrence, E.C.; Levine, K.L.; Dabovic, B.; Jung, C.; Davis, E.C.; Madan-Khetarpal, S.; Urban, Z. Latent transforming growth factor binding protein 4 regulates transforming growth factor beta receptor stability. Hum. Mol. Genet. 2015, 24, 4024–4036.
- Lu, J.; Liu, Q.; Wang, L.; Tu, W.; Chu, H.; Ding, W.; Jiang, S.; Ma, Y.; Shi, X.; Pu, W.; et al. Increased expression of latent TGF-beta-binding protein 4 affects the fibrotic process in scleroderma by TGF-beta/SMAD signaling. Lab. Investig. 2017, 97, 591–601.
- Shi, M.; Zhu, J.; Wang, R.; Chen, X.; Mi, L.; Walz, T.; Springer, T.A. Latent TGF-beta structure and activation. Nature 2011, 474, 343–349.
- Buscemi, L.; Ramonet, D.; Klingberg, F.; Formey, A.; Smith-Clerc, J.; Meister, J.J.; Hinz, B. The single-molecule mechanics of the latent TGF-beta1 complex. Curr. Biol. 2011, 21, 2046–2054.
- Campbell, M.G.; Cormier, A.; Ito, S.; Seed, R.I.; Bondesson, A.J.; Lou, J.; Marks, J.D.; Baron, J.L.; Cheng, Y.; Nishimura, S.L. Cryo-EM Reveals Integrin-Mediated TGF-beta Activation without Release from Latent TGF-beta. Cell 2020, 180, 490–501.e16.
- Isogai, Z.; Ono, R.N.; Ushiro, S.; Keene, D.R.; Chen, Y.; Mazzieri, R.; Charbonneau, N.L.; Reinhardt, D.P.; Rifkin, D.B.; Sakai, L.Y. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J. Biol. Chem. 2003, 278, 2750–2757.
- Neptune, E.R.; Frischmeyer, P.A.; Arking, D.E.; Myers, L.; Bunton, T.E.; Gayraud, B.; Ramirez, F.; Sakai, L.Y.; Dietz, H.C. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003, 33, 407–411.
- Nistala, H.; Lee-Arteaga, S.; Smaldone, S.; Siciliano, G.; Carta, L.; Ono, R.N.; Sengle, G.; Arteaga-Solis, E.; Levasseur, R.; Ducy, P.; et al. Fibrillin-1 and -2 differentially modulate endogenous TGF-beta and BMP bioavailability during bone formation. J. Cell. Biol. 2010, 190, 1107–1121.
- Zeyer, K.A.; Zhang, R.M.; Kumra, H.; Hassan, A.; Reinhardt, D.P. The Fibrillin-1 RGD Integrin Binding Site Regulates Gene Expression and Cell Function through microRNAs. J. Mol. Biol. 2019, 431, 401–421.
- Ramnath, N.W.; Hawinkels, L.J.; van Heijningen, P.M.; te Riet, L.; Paauwe, M.; Vermeij, M.; Danser, A.H.; Kanaar, R.; ten Dijke, P.; Essers, J. Fibulin-4 deficiency increases TGF-beta signalling in aortic smooth muscle cells due to elevated TGF-beta2 levels. Sci. Rep. 2015, 5, 16872.
- Burger, J.; van Vliet, N.; van Heijningen, P.; Kumra, H.; Kremers, G.J.; Alves, M.; van Cappellen, G.; Yanagisawa, H.; Reinhardt, D.P.; Kanaar, R.; et al. Fibulin-4 deficiency differentially affects cytoskeleton structure and dynamics as well as TGFbeta signaling. Cell Signal. 2019, 58, 65–78.
- Kuang, P.P.; Joyce-Brady, M.; Zhang, X.H.; Jean, J.C.; Goldstein, R.H. Fibulin-5 gene expression in human lung fibroblasts is regulated by TGF-beta and phosphatidylinositol 3-kinase activity. Am. J. Physiol. Cell Physiol. 2006, 291, C1412–C1421.
- Lee, Y.H.; Albig, A.R.; Regner, M.; Schiemann, B.J.; Schiemann, W.P. Fibulin-5 initiates epithelial-mesenchymal transition (EMT) and enhances EMT induced by TGF-beta in mammary epithelial cells via a MMP-dependent mechanism. Carcinogenesis 2008, 29, 2243–2251.
- Topalovski, M.; Hagopian, M.; Wang, M.; Brekken, R.A. Hypoxia and Transforming Growth Factor beta Cooperate to Induce Fibulin-5 Expression in Pancreatic Cancer. J. Biol. Chem. 2016, 291, 22244–22252.
- Schiemann, W.P.; Blobe, G.C.; Kalume, D.E.; Pandey, A.; Lodish, H.F. Context-specific effects of fibulin-5 (DANCE/EVEC) on cell proliferation, motility, and invasion. Fibulin-5 is induced by transforming growth factor-beta and affects protein kinase cascades. J. Biol. Chem. 2002, 277, 27367–27377.
- Lee, S.S.; Knott, V.; Jovanović, J.; Harlos, K.; Grimes, J.M.; Choulier, L.; Mardon, H.J.; Stuart, D.I.; Handford, P.A. Structure of the integrin binding fragment from fibrillin-1 gives new insights into microfibril organization. Structure 2004, 12, 717–729.
- Pfaff, M.; Reinhardt, D.P.; Sakai, L.Y.; Timpl, R. Cell adhesion and integrin binding to recombinant human fibrillin-1. FEBS Lett. 1996, 384, 247–250.
- Bax, D.V.; Bernard, S.E.; Lomas, A.; Morgan, A.; Humphries, J.; Shuttleworth, C.A.; Humphries, M.J.; Kielty, C.M. Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by alpha 5 beta 1 and alpha v beta 3 integrins. J. Biol. Chem. 2003, 278, 34605–34616.
- Jovanovic, J.; Takagi, J.; Choulier, L.; Abrescia, N.G.; Stuart, D.I.; van der Merwe, P.A.; Mardon, H.J.; Handford, P.A. alphaVbeta6 is a novel receptor for human fibrillin-1. Comparative studies of molecular determinants underlying integrin-rgd affinity and specificity. J. Biol. Chem. 2007, 282, 6743–6751.
- Del, C.J.; Reed, N.I.; Molnar, K.; Liu, S.; Dang, B.; Jensen, S.A.; DeGrado, W.; Handford, P.A.; Sheppard, D.; Sundaram, A.B. A disease-associated mutation in fibrillin-1 differentially regulates integrin-mediated cell adhesion. J. Biol. Chem. 2019, 294, 18232–18243.
- Bax, D.V.; Rodgers, U.R.; Bilek, M.M.; Weiss, A. Cell adhesion to tropoelastin is mediated via the C-terminal GRKRK motif and integrin alphaVbeta3. J. Biol. Chem. 2009, 284, 28616–28623.
- Lee, P.; Bax, D.V.; Bilek, M.M.; Weiss, A.S. A novel cell adhesion region in tropoelastin mediates attachment to integrin alphaVbeta5. J. Biol. Chem. 2014, 289, 1467–1477.
- Bochicchio, B.; Yeo, G.C.; Lee, P.; Emul, D.; Pepe, A.; Laezza, A.; Ciarfaglia, N.; Quaglino, D.; Weiss, A.S. Domains 12 to 16 of tropoelastin promote cell attachment and spreading through interactions with glycosaminoglycan and integrins alphaV and alpha5beta1. FEBS J. 2021, 288, 4024–4038.
- Ozsvar, J.; Wang, R.; Tarakanova, A.; Buehler, M.J.; Weiss, A.S. Fuzzy binding model of molecular interactions between tropoelastin and integrin alphaVbeta3. Biophys. J. 2021, 120, 3138–3151.
- Lomas, A.C.; Mellody, K.T.; Freeman, L.J.; Bax, D.V.; Shuttleworth, C.A.; Kielty, C.M. Fibulin-5 binds human smooth-muscle cells through alpha5beta1 and alpha4beta1 integrins, but does not support receptor activation. Biochem. J. 2007, 405, 417–428.
- Furie, N.; Shteynberg, D.; Elkhatib, R.; Perry, L.; Ullmann, Y.; Feferman, Y.; Preis, M.; Flugelman, M.Y.; Tzchori, I. Fibulin-5 regulates keloid-derived fibroblast-like cells through integrin beta-1. Int. J. Cosmet. Sci. 2016, 38, 35–40.
