Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Breast implant illness refers to a combination of different symptoms related to breast implant surgery, including fatigue, brain fog, and arthralgias. This malaise occurs after cosmetic and reconstructive breast surgeries, although it has not been proven to be a disease. Complications associated with breast implants include breast pain, capsular contracture, infections, as well as other manifestations specific to breast reconstruction.
- BII
- breast
- implant
- illness
- surgery
- autoimmunity
- plastic surgery
- silicone
1. Introduction
Outstanding advancements have been made in the medical field since the inception of synthetic prosthetics [1][2]. Presently, prosthetics have changed disease therapeutics and improved the quality of life for many patients [3]. Globally, a portion of the medical community has been skeptical about the use and safety of cosmetic silicone breast implants (SBI) [4]. This is mainly due to the absence of physiologic function and the immense popularity of breast surgery worldwide, with 287,085 interventions in 2019 and 193,073 in 2020 in the United States alone [5].
New concerns regarding the use of SBI have emerged apart from infection and immune rejection [6]. Asymmetry, breast pain, capsular contracture, implant rupture, infections, and breast implant-associated anaplastic large cell lymphoma have been reported to date [7][8]. Particularly, patients have reported symptoms of new-onset autoimmune disorder (18%), depression (19%), hair loss (21%), anxiety (24%), arthralgias (25%), brain fog (25%), and fatigue (49%) after SBI surgery, leading to a new term being coined for this syndrome: breast implant illness (BII) [9][10].
2. Surgical Implications
One standard type of SBI and surgical planning cannot be used for all, and the choice is usually based on the surgeon’s experience [11]. Correspondingly, SBI placement involves several incision sites that appear as axillary, inframammary, periareolar, and mastectomy scars [11][12] (Figure 1). Moreover, each incision site provides different degrees of tissue manipulation [13].
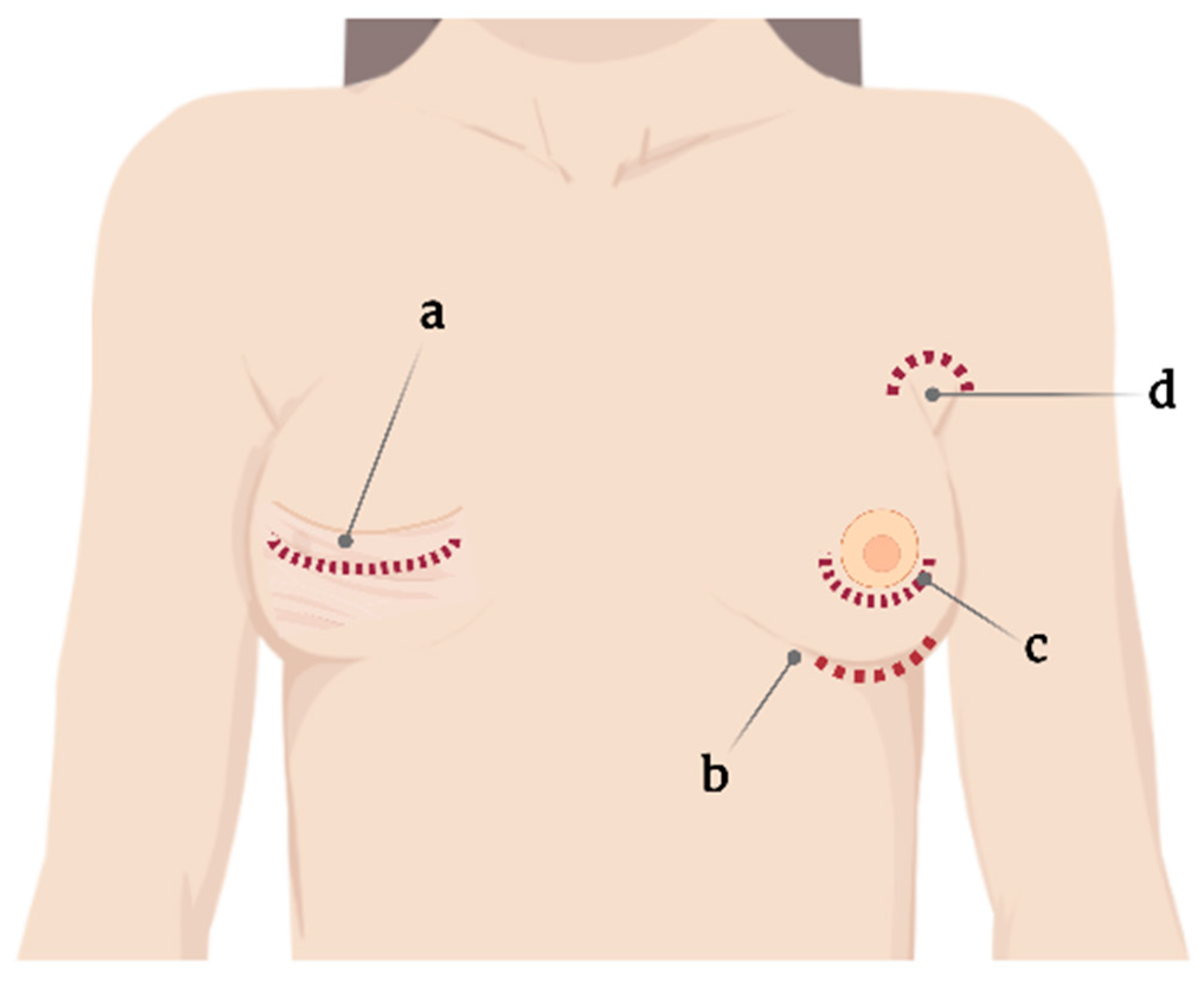
Figure 1. Current incision sites for silicone breast implant placement. (a) Mastectomy scar, (b) submammary, (c) periareolar, and (d) axillary.
Specifically, the periareolar incision site shows a higher incidence of capsular contracture (6.9%) compared with inframammary incision (4.5%) [14]. When using such an approach, large ducts containing endogenous bacteria are dissected, resulting in potential implant contamination, subsequently leading to biofilm formation and further capsular contracture [15].
2.1. Capsular Contracture
Capsular contracture is one of the most common complications after breast implant surgery (Figure 2) [16]. Currently, according to Baker staging, capsular contracture can be of four degrees [17] and is believed to be caused by leukocytic infiltration and fibroblast proliferation around the implant [18]. Capsular contracture is observed in up to 20% of patients with SBI, with patients with a body mass index of >30 kg/m2 and undergoing complete breast reconstructions being at increased risk [19][20].
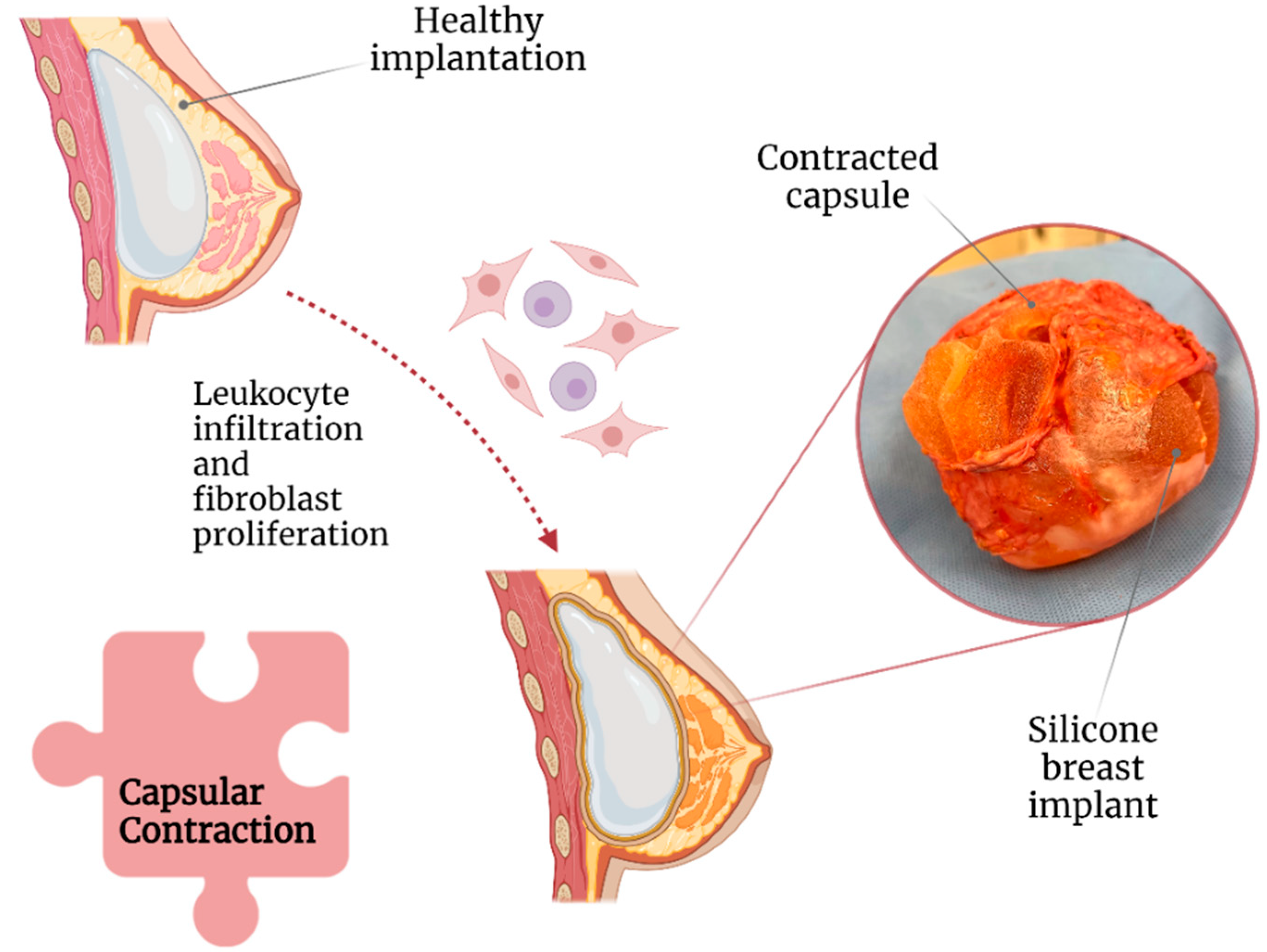
Figure 2. Association of capsular contraction with breast implant illness.
Moreover, based on its stage, capsular contracture can result in significant breast pain, deformity, and induration [21]. A study revealed that capsular contracture and BII were the cause of explantation in 14.7% and 11.2% of 197 patients, respectively [22]. Correspondingly, a meta-analysis concluded that the following factors could play a role in capsular contracture development: (1) breast reconstructive surgery, (2) postoperative hematoma, and (3) axillary surgical approach. All of them are possibly due to increased tissue manipulation and dissection [11].
Capsular contracture is more common in submammary implants, perhaps because of insufficient barrier protection from the pectoral muscle against local bacteria [11][23]. Some studies have suggested that the degree of capsular contracture is correlated with capsular thickness, but this remains controversial [24][25]. In terms of the texture of the SBI, a follow-up study including 2560 patients revealed an adjusted relative risk of 2.25 (95% confidence interval) for capsular contracture in smooth implants compared with textured ones [23]. Similarly, microtextured implants have lower rates of capsular contracture compared with macrotextured ones [26].
2.2. Texture of SBI and Anaplastic Large Cell Lymphoma
Textured implants were introduced around 1970 in an attempt to reduce the incidence of capsular contracture [25]. Among the micropatterning techniques that were used, imprinted textured SBI has had the lowest rate of capsular contracture (3.8%) compared with foam (4.9%), salt-loss textured (5.27%), and smooth (15.5%) SBIs [27].
Nonetheless, compared with smooth SBI, a textured SBI has been highly associated with breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) [28] (Figure 3).
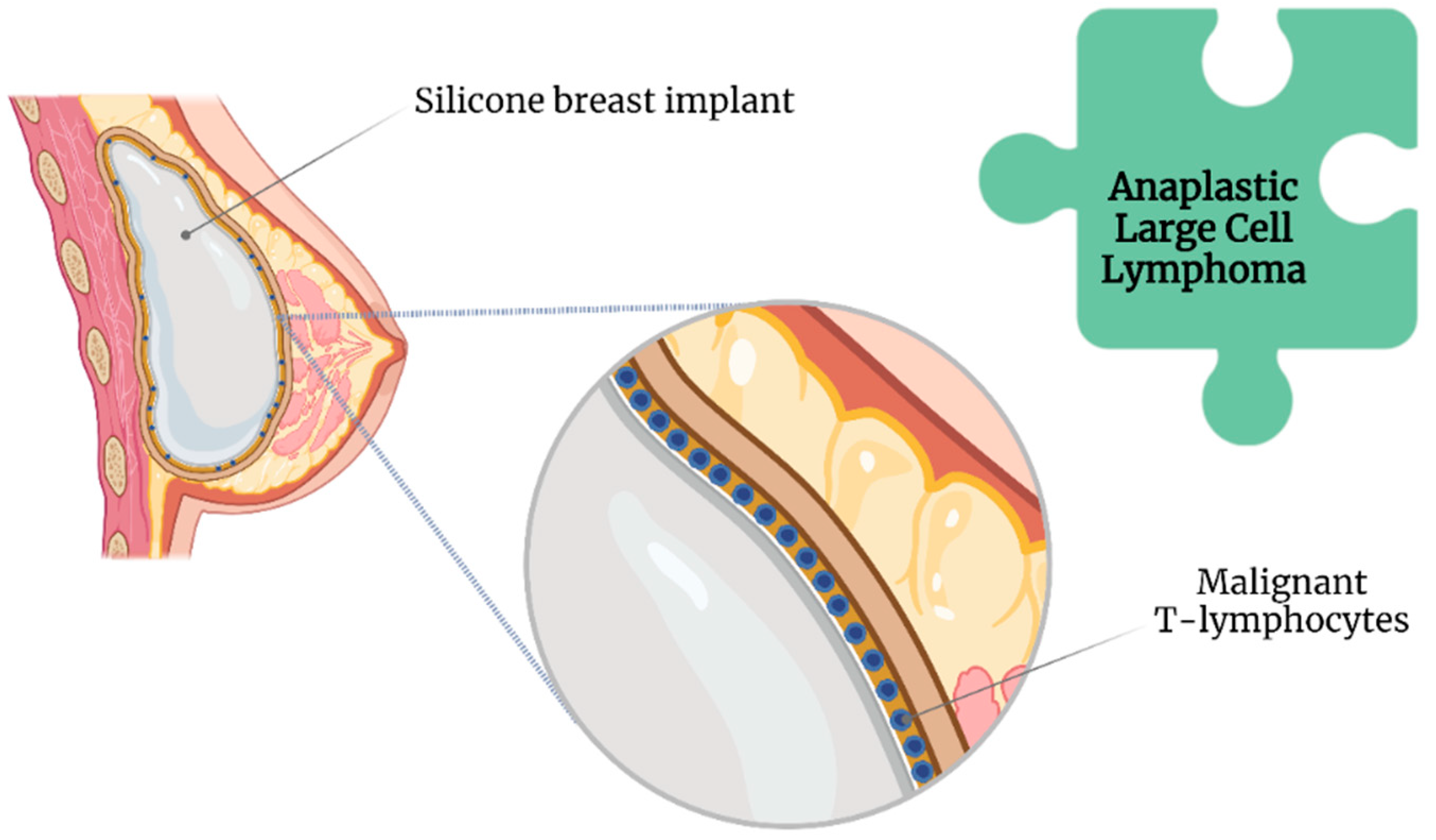
Figure 3. Association of anaplastic large cell lymphoma with breast implant illness.
The Food and Drug Administration has requested the withdrawal of all Biocell textured SBI because of this association [29]. Likewise, Allergan has issued a global recall of their implants [29]. However, the lifelong risk of developing BII ranges between 0.0017 and 0.028, even with an increased incidence of BIA-ALCL [30].
Although routinely performed, en bloc capsulectomy, consisting of complete SBI removal within the capsule along with a margin of healthy tissue [31], is only recommended in the presence of BIA-ALCL [32]. This recommendation is given because of the reported invasiveness as well as increased surgical risk, discomfort, recovery time, and the absence of proven additional benefits in patients with mild symptoms [33].
Furthermore, no current evidence indicates that a prophylactic capsulectomy reduces the risk of BIA-ALCL [34].
2.3. Biofilms
By definition, biofilms are colonies of one or more types of microorganisms living in symbiosis [35][36]. Unlike bacteremia, a biofilm does not present with the classic symptoms of infection as it induces a different immunological response [37]; this is more worrisome in the long term, as it involves chronic and significant immunological activation [38] unless the patient is immunosuppressed [39].
Biofilms further protect bacteria from host immune responses and antimicrobial therapy [40]. Extracellular insoluble polysaccharides secreted by microorganisms allow the adhesion of biofilms to the surface of prosthetic materials, such as SBI (Figure 4) [41]. However, biofilms are not exclusive to SBI [42].
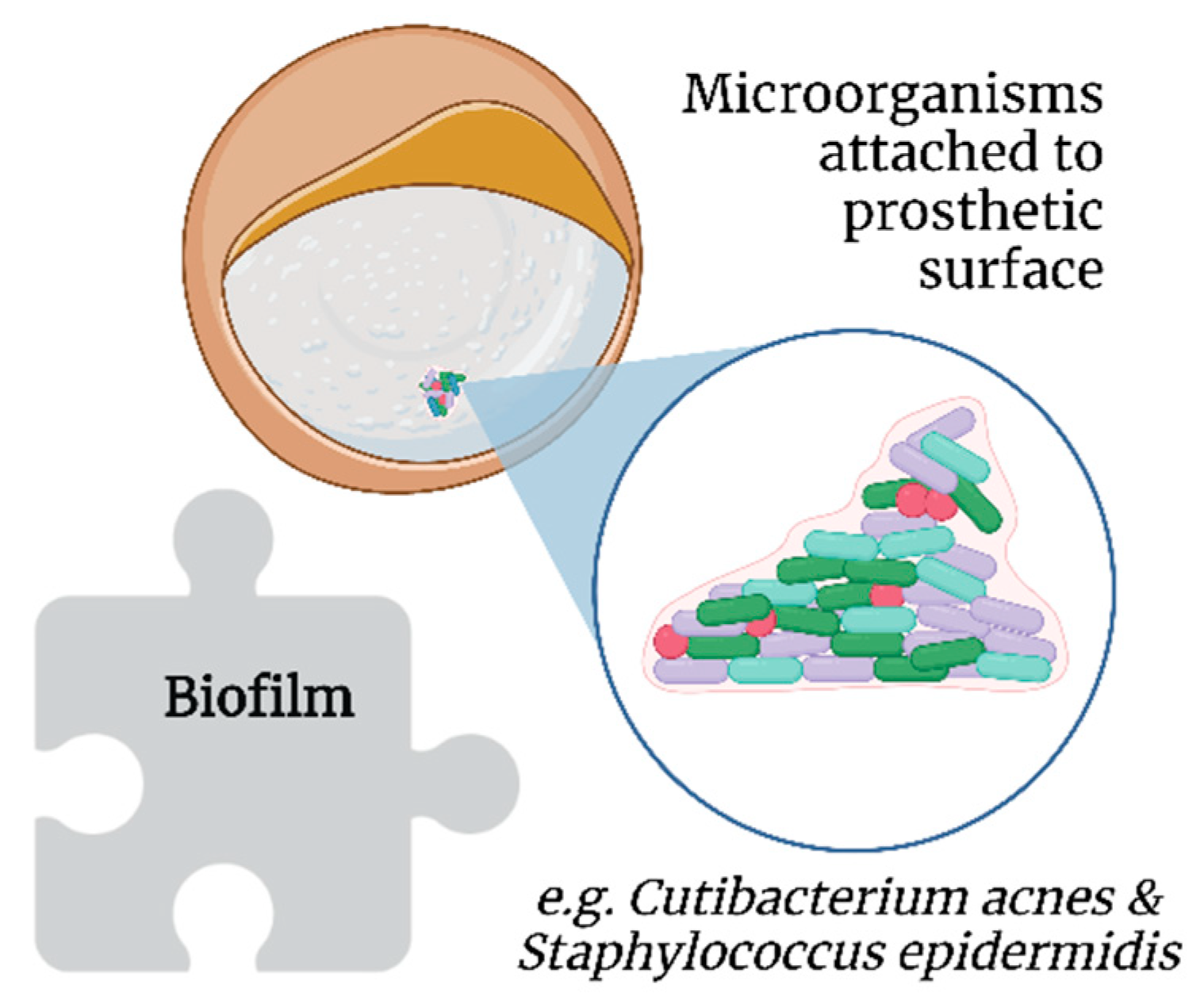
Figure 4. Association of biofilms with breast implant illness.
Cutibacterium acnes (formerly Propionibacterium acnes) and Staphylococcus epidermidis, which are part of breast microbiota, are common in implant biofilms [43]. Additionally, thicker biofilms have been observed on textured SBI in vitro, probably because of the increased surface area compared with smooth SBI [44]. Moreover, C. acnes has been posited to contribute to the development of BII, as its presence allows for persistent low-grade inflammation and resultant capsular contracture, which are two mechanisms proposed as key factors in the genesis and progression of BII [45].
Two comparative clinical trials reported a 10-fold decrease in capsular contracture with the use of antibiotic or antiseptic pocket irrigation during cosmetic breast surgeries, thus supporting the role of biofilms in capsular contracture [14][46][47]. Further, povidone-iodine [26] and chlorhexidine gluconate have been shown to reduce biofilm-related capsular contracture [48][49].
2.4. Infections
As in many other circumstances, the introduction of foreign material into the body increases the risk of infection [42]. Specifically, infections are reported in 2.6% of SBI surgeries, predominantly because of inoculation during the surgical procedure, followed by hematogenous or contiguous spread [11][13].
Several authors have reported chronic infections in SBI capsules [16][35][48]. Such infections can cause capsule growth and further contraction [50]. Chronic infections have been posited to play a role in the genesis of breast implant-associated anaplastic large-cell lymphoma [24][51].
Patients with chronically infected capsules or synovial metaplasia reportedly have the best symptom resolution after explantation [50]. Notwithstanding, false-negative results account for 30% of cases in the field of SBI swab testing [52]. Thus, techniques such as extended culturing protocols and sonication have been suggested [53], which have shown 20% higher sensitivity (81% vs. 61%) and 99% specificity for bacterial detection in other prosthetic infections compared to simple culturing techniques [52][54].
3. Autoimmune Responses
SBI surgery requires a specialized and highly detailed procedure that must consider certain biological aspects, including BII [19]. The role of autoimmune responses on the implant is a major concern associated with to BII [55][56][57][58]. Nevertheless, some authors are of the opinion that the term BII must be included with other autoimmune disorders or functional somatic syndromes [56][59][60][61].
3.1. The Immune Response to SBI
Somatic syndromes are predominantly characterized by subjective symptoms rather than clinical signs by a physician, such as tissue abnormalities or specific physical findings [59][61][62]. Frequent patient-reported symptoms of brain fog, mood disturbances, xerophthalmia, fever, paresthesia, and arthralgia, among others, after SBI surgery have led authors to coin the term “siliconosis” or “silicone reactive disorder” [19][56][61][63][64][65][66].
However, other authors posit these symptoms represent a subtype of an adjuvant-induced autoimmune syndrome, a broad term that includes BII and other implant-associated diseases [56][65], termed silicone implant incompatibility syndrome (SIIS) [67][68].
SIIS is presumed to share pathophysiological features with fibromyalgia [69]. The nociceptive stimulus (silicone) combined with extensive worries about the safety of the SBI can lead to disturbances in pain signaling pathways, with excessive neurotransmitter stimulation resulting in systemic complaints [70]. Additionally, cytokines released during chronic inflammation may lead to persistent alterations in dopaminergic pathways and basal ganglia, leading to persistent anhedonia, fatigue, and psychomotor slowing [71].
Silicone particles can be shed from the textured implants, leading to a chronic immunological stimulus in the body [33] (Figure 5). This theory is supported by the findings in the lymphoid tissue and thymus related to silicone-activated inflammasomes, cytokine production, and neutrophil and macrophage recruitment, even leading to granulomas [58][72].
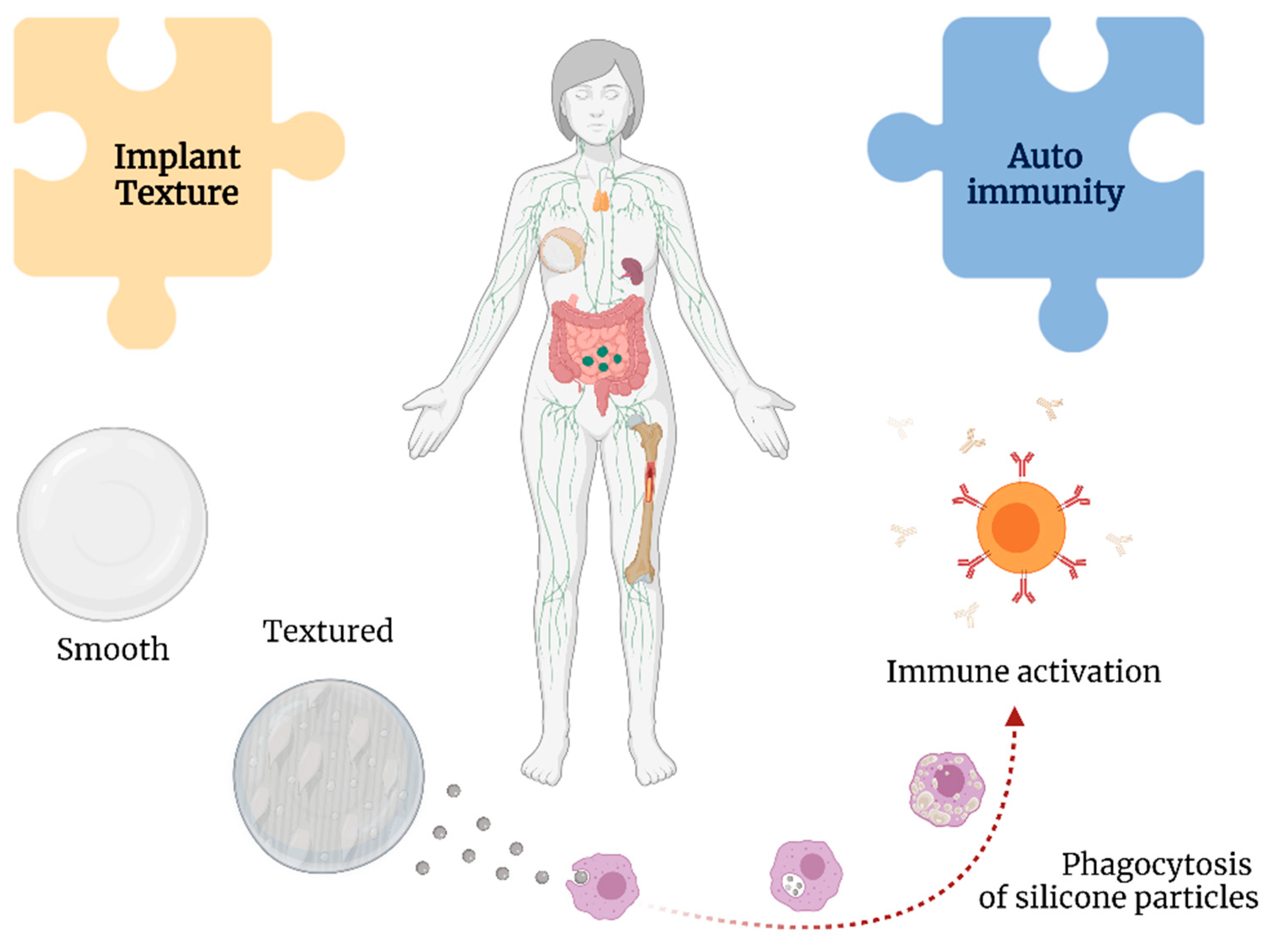
Figure 5. Associations between implant texture and autoimmunity with breast implant illness.
Shedding from the implant may result in the formation of silicone-induced granuloma of the breast implant capsule. This condition is posited to represent autoimmune syndrome induced by adjuvants (ASIA), with the adjuvants in this instance being silicone [73]. This leads to the activation of macrophages and increased levels of interleukin-1β and reactive oxygen and nitrogen species [74].
Moreover, silicone particles can elicit a type 2 inflammatory response and increase levels of immunoglobulins G1 and E [75], resulting in the chronic activation of fibroblasts, myofibroblasts, and T lymphocytes contributing to increased collagen deposition and, ultimately, capsular contracture [76].
The surface charge of nanoparticles may play an important role in their cytotoxicity [77]. Silicone particles have been shown to decrease phagocytosis, induce the production of intracellular reactive oxygen species, and reduce mitochondrial metabolic activities [78]. Multiple studies have demonstrated the development of autoantibodies in response to silicone implants, such as anti-nuclear antibodies and rheumatoid factor, suggesting a persistent antigenic role of SBI [70][79].
These findings are highly suggestive of silicone lymphadenopathy, a condition that pathologically resembles and is often misdiagnosed as cancer or other immune-mediated pathologies [56][80][81][82][83]. Research has also suggested that ASIA involves an interplay between immunogenetic factors, such as human leukocyte agent, as well as environmental factors, such as smoking and obesity [84].
3.2. Somatic Syndromes
A number of other functional somatic syndromes with an unclear etiology share common symptoms with BII, such as chronic fatigue syndrome and fibromyalgia [59][60]. A previous study demonstrated that the presence of these syndromes as well as a chronic disease and advanced age were independent predictors for the development of BII-related symptoms [55].
The role of SBI in the genesis or worsening of connective tissue disorders has been previously reported [64]. Specifically, a study including 220 women with SBI and 879 women without SBI reported a relative risk of 1.24 for any connective tissue diseases in women with SBI compared with the general population [85]. Moreover, the relative risk for explantation was 5.6-fold higher in patients meeting the major criteria for adjuvant-induced autoimmune syndrome and 4.3-fold higher for those meeting the minor criteria [86].
3.3. Explantation and Reported Symptoms of BII
Rohrich et al. reported improved musculoskeletal symptoms, mental health, and body image after explantation [87]. Likewise, de Boer et al. demonstrated improvements in symptoms of fatigue, arthralgia, and myalgia as well as in memory and sleep disturbances in 76% of patients after explantation [88]. A recent study by Miseré and van der Hulst reported subjective improvements in complaints in 60% of patients [22]. Similar findings have been reported by other authors as well [61][89][90][91][92][93][94][95], suggesting that a decreased inflammatory response and elimination of the nociceptive stimulus explains the improvements in highly symptomatic patients after explantation [87][88].
Another study revealed that only patients with elevated serologic markers of autoimmune disorders experienced a short-lived improvement in symptoms after explantation; however, symptoms recurred within the first 6–12 months postoperatively, whereas patients with a previously diagnosed autoimmune disorder showed no improvement in symptoms [62][95]. These findings are supported by a recent systematic review [88].
This entry is adapted from the peer-reviewed paper 10.3390/surgeries3020013
References
- Nahabedian, M.Y. Innovations and advancements with prosthetic breast reconstruction. Breast J. 2018, 24, 586–591.
- Harvey, Z.T.; Potter, B.K.; Vandersea, J.; Wolf, E. Prosthetic advances. J. Surg. Orthop. Adv. 2012, 21, 58–64.
- Arabian, A.; Varotsis, D.; McDonnell, C.; Meeks, E. Global social acceptance of prosthetic devices. In Proceedings of the GHTC 2016—2016 IEEE Global Humanitarian Technology Conference (GHTC) Technology for the Benefit of Humanity Conference Proceedings, Seattle, WA, USA, 13–16 October 2016; Institute of Electrical and Electronics Engineers Inc.: New York, NY, USA, 2016; pp. 563–568.
- Son, W.J.; Kang, S.G.; Seo, B.F.; Choi, N.-K. A Systematic Review of the National Breast Implant Registry for Application in Korea: Can We Predict “Unpredictable” Complications? Medicina 2020, 56, 370.
- American Society of Plastic Surgeons. Plastic Surgery Statistics Report 2020—ASPS National Clearinghouse of Plastic Surgery Procedural Statistics; SPS: West Valley City, UT, USA, 2021; pp. 1–26.
- U. S. Food and Drug Administration. Risks and Complications of Breast Implants Breast Implants—Certain Labeling Recommendations to Improve; FDA; Silver Spring: Montgomery County, MD, USA, 2021; pp. 1–10.
- Vasey, F.B.; Zarabadi, S.A.; Seleznick, M.; Ricca, L. Where there’s smoke there’s fire: The silicone breast implant controversy continues to flicker: A new disease that needs to be defined. J. Rheumatol. 2003, 30, 2092–2094.
- Bouhadana, G.; Chocron, Y.; Azzi, A.J.; Davison, P.G. Perception of implants among breast reconstruction patients in montreal. Plast. Reconstr. Surg.—Glob. Open. 2020, 8, e3116.
- Siling, Y.; Klietz, M.-L.; Harren, A.K.; Wei, Q.; Hirsch, T.; Aitzetmüller, M.M. Understanding Breast Implant Illness: Etiology is the Key. Aesthet. Surg. J. 2021, 42, 370–377.
- Tang, S.Y.Q.; Israel, J.S.; Afifi, A.M. Breast Implant Illness: Symptoms, Patient Concerns, and the Power of Social Media. Plast. Reconstr. Surg. 2017, 140, 765e–766e.
- Bachour, Y.; Bargon, C.A.; de Blok, C.J.M.; Ket, J.C.F.; Ritt, M.J.P.F.; Niessen, F.B. Risk factors for developing capsular contracture in women after breast implant surgery: A systematic review of the literature. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, e29–e48.
- Jacobson, J.M.; Gatti, M.E.; Schaffner, A.D.; Hill, L.M.; Spear, S.L. Effect of incision choice on outcomes in primary breast augmentation. Aesthet. Surg. J. 2012, 32, 456–462.
- Li, S.; Chen, L.; Liu, W.; Mu, D.; Luan, J. Capsular Contracture Rate After Breast Augmentation with Periareolar Versus Other Two (Inframammary and Transaxillary) Incisions: A Meta-Analysis. Aesthet. Plast. Surg. 2018, 42, 32–37.
- Blount, A.L.; Martin, M.D.; Lineberry, K.D.; Kettaneh, N.; Alfonso, D.R. Capsular contracture rate in a low-risk population after primary augmentation mammaplasty. Aesthet. Surg. J. 2013, 33, 516–521.
- Wiener, T.C. Relationship of incision choice to capsular contracture. Aesthet. Plast. Surg. 2008, 32, 303–306.
- Galdiero, M.; Larocca, F.; Iovene, M.R.; Francesca, M.; Pieretti, G.; D’Oriano, V.; Franci, G.; Ferraro, G.; D’Andrea, F.; Nicoletti, G.F. Microbial Evaluation in Capsular Contracture of Breast Implants. Plast. Reconstr. Surg. 2018, 141, 23–30.
- Prantl, L.; Angele, P.; Schreml, S.; Ulrich, D.; Pöppl, N.; Eisenmann-Klein, M. Determination of serum fibrosis indexes in patients with capsular contracture after augmentation with smooth silicone gel implants. Plast. Reconstr. Surg. 2006, 118, 224–229.
- Sood, A.; Xue, E.Y.; Sangiovanni, C.; Therattil, P.J.; Lee, E.S. Breast Massage, Implant Displacement, and Prevention of Capsular Contracture After Breast Augmentation With Implants: A Review of the Literature. Eplasty 2017, 17, e41.
- Wee, C.E.; Younis, J.; Isbester, K.; Smith, A.; Wangler, B.; Sarode, A.L.; Patil, N.; Grunzweig, K.; Boas, S.; Harvey, D.J.; et al. Understanding Breast Implant Illness, Before and After Explantation: A Patient-Reported Outcomes Study. Ann. Plast. Surg. 2020, 85, S82–S86.
- Calobrace, M.B.; Schwartz, M.R.; Zeidler, K.R.; Pittman, T.A.; Cohen, R.; Stevens, W.G. Long-term safety of textured and smooth breast implants. Aesthet. Surg. J. 2018, 38, 38–48.
- Hammond, J.B.; Kosiorek, H.E.; Cronin, P.A.; Rebecca, A.M.; Casey, W.J.; Wong, W.W.; Vargas, C.E.; Vern-Gross, T.Z.; McGee, L.A.; Pockaj, B.A. Capsular contracture in the modern era: A multidisciplinary look at the incidence and risk factors after mastectomy and implant-based breast reconstruction. Am. J. Surg. 2021, 221, 1005–1010.
- Miseré, R.M.L.; van der Hulst, R.R.W.J. Self-Reported Health Complaints in Women Undergoing Explantation of Breast Implants. Aesthet. Surg. J. 2022, 42, 171–180.
- Stevens, W.G.; Nahabedian, M.Y.; Calobrace, M.B.; Harrington, J.L.; Capizzi, P.J.; Cohen, R.; D’incelli, R.C.; Beckstrand, M. Risk factor analysis for capsular contracture: A 5-year sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast. Reconstr. Surg. 2013, 132, 1115–1123.
- Jones, P.; Mempin, M.; Hu, H.; Chowdhury, D.; Foley, M.; Cooter, R.; Adams, W.P.; Vickery, K.; Deva, A.K. The functional influence of breast implant outer shell morphology on bacterial attachment and growth. Plast. Reconstr. Surg. 2018, 142, 837–849.
- Munhoz, A.M.; Clemens, M.W.; Nahabedian, M.Y. Breast Implant Surfaces and Their Impact on Current Practices. Plast. Reconstr. Surg.—Glob. Open 2019, 7, e2466.
- Luvsannyam, E.; Patel, D.; Hassan, Z.; Nukala, S.; Somagutta, M.R.; Hamid, P. Overview of Risk Factors and Prevention of Capsular Contracture Following Implant-Based Breast Reconstruction and Cosmetic Surgery: A Systematic Review. Cureus 2020, 12, e10341.
- Shauly, O.; Gould, D.J.; Patel, K.M. Microtexture and the cell/biomaterial interface: A systematic review and meta-analysis of capsular contracture and prosthetic breast implants. Aesthet. Surg. J. 2019, 39, 603–614.
- Turner, S.D.; Inghirami, G.; Miranda, R.N.; Kadin, M.E. Cell of Origin and Immunologic Events in the Pathogenesis of Breast Implant–Associated Anaplastic Large-Cell Lymphoma. Am. J. Pathol. 2020, 190, 2–10.
- Swanson, E. The Food and Drug Administration Bans Biocell Textured Breast Implants: Lessons for Plastic Surgeons. Ann. Plast. Surg. 2020, 84, 343–345.
- McKernan, C.D.; Vorstenbosch, J.; Chu, J.J.; Nelson, J.A. Breast Implant Safety: An Overview of Current Regulations and Screening Guidelines. J. Gen. Intern. Med. 2022, 37, 212–216.
- Johal, K.S.; Floyd, D. To bloc or not to bloc: Challenges in the management of patients requesting “En-Bloc capsulectomy”. Aesthet. Surg. J. 2020, 40, NP561–NP563.
- Keane, G.; Chi, D.; Ha, A.Y.; Myckatyn, T.M. En Bloc Capsulectomy for Breast Implant Illness: A Social Media Phenomenon? Aesthet. Surg. J. 2021, 41, 448–459.
- Swanson, E. Evaluating the necessity of capsulectomy in cases of textured breast implant replacement. Ann. Plast. Surg. 2020, 85, 691–698.
- Abi-Rafeh, J.; Safran, T.; Winocour, S.; Dionisopoulos, T.; Davison, P.; Vorstenbosch, J. Lack of Evidence on Complication Profile of Breast Implant Capsulectomy: A Call to Action for Plastic Surgeons. Plast. Reconstr. Surg. 2021, 148, 157e–158e.
- Ajdic, D.; Zoghbi, Y.; Gerth, D.; Panthaki, Z.J.; Thaller, S. The relationship of bacterial biofilms and capsular contracture in breast implants. Aesthet. Surg. J. 2016, 36, 297–309.
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015, 10, 1743–1750.
- Nguyen, T.H.; Park, M.D.; Otto, M. Host response to Staphylococcus epidermidis colonization and infections. Front. Cell. Infect. Microbiol. 2017, 7, 90.
- Beam, E.; Osmon, D. Prosthetic Joint Infection Update. Infect. Dis. Clin. N. Am. 2018, 32, 843–859.
- Dowsett, C.; Bellingeri, A.; Carville, K.; Garten, A.; Woo, K. A route to more effective infection management: The Infection Management Pathway. Wounds Int. 2020, 11, 50–57.
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512.
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 1083–1099.
- Fernandez-Moure, J.S.; Mydlowska, A.; Shin, C.; Vella, M.; Kaplan, L.J. Nanometric Considerations in Biofilm Formation. Surg. Infect. 2019, 20, 167–173.
- Achermann, Y.; Goldstein, E.J.C.; Coenye, T.; Shirtliffa, M.E. Propionibacterium acnes: From Commensal to opportunistic biofilm-associated implant pathogen. Clin. Microbiol. Rev. 2014, 27, 419–440.
- James, G.A.; Boegli, L.; Hancock, J.; Bowersock, L.; Parker, A.; Kinney, B.M. Bacterial Adhesion and Biofilm Formation on Textured Breast Implant Shell Materials. Aesthet. Plast. Surg. 2019, 43, 490–497.
- McCarthy, P.H.; Teitler, N.A.; Hon, H.H.; Miller, J.J. Breast Implant Illness and Cutibacterium acnes: A Case Report. Plast. Reconstr. Surg.—Glob. Open 2022, 10, E4146.
- Mempin, M.; Hu, H.; Chowdhury, D.; Deva, A.; Vickery, K. The A, B and C’s of silicone breast implants: Anaplastic large cell lymphoma, biofilm and capsular contracture. Materials 2018, 11, 2393.
- Giordano, S.; Peltoniemi, H.; Lilius, P.; Salmi, A. Povidone-iodine combined with antibiotic topical irrigation to reduce capsular contracture in cosmetic breast augmentation: A comparative study. Aesthet. Surg. J. 2013, 33, 675–680.
- Carvajal, J.; Carvajal, M.; Hernández, G. Back to Basics: Could the Preoperative Skin Antiseptic Agent Help Prevent Biofilm-Related Capsular Contracture? Aesthet. Surg. J. 2019, 39, 848–859.
- Yalanis, G.C.; Liu, E.W.; Cheng, H.T. Efficacy and safety of povidone-iodine irrigation in reducing the risk of capsular contracture in aesthetic breast augmentation: A systematic review and meta-analysis. Plast. Reconstr. Surg. 2015, 136, 687–698.
- Lee, M.; Ponraja, G.; McLeod, K.; Chong, S. Breast Implant Illness: A Biofilm Hypothesis. Plast. Reconstr. Surg.—Glob. Open 2020, 8, e2755.
- Loch-Wilkinson, A.; Beath, K.J.; Knight, R.J.W.; Wessels, W.L.F.; Magnusson, M.; Papadopoulos, T.; Connell, T.; Lofts, J.; Locke, M.; Hopper, I.; et al. Breast implant-associated anaplastic large cell lymphoma in Australia and New Zealand: High-surface-area textured implants are associated with increased risk. Plast. Reconstr. Surg. 2017, 140, 645–654.
- Rieger, U.M.; Pierer, G.; Lüscher, N.J.; Trampuz, A. Sonication of removed breast implants for improved detection of subclinical infection. Aesthet. Plast. Surg. 2009, 33, 404–408.
- Schäfer, P.; Fink, B.; Sandow, D.; Margull, A.; Berger, I.; Frommelt, L. Prolonged bacterial culture to identify late periprosthetic joint infection: A promising strategy. Clin. Infect. Dis. 2008, 47, 1403–1409.
- Portillo, M.E.; Salvadó, M.; Alier, A.; Martínez, S.; Sorli, L.; Horcajada, J.P.; Puig, L. Advantages of sonication fluid culture for the diagnosis of prosthetic joint infection. J. Infect. 2014, 69, 35–41.
- Miseré, R.; Maartje, C.; van der Hulst, R.R.W.J. The Prevalence of Self-Reported Health Complaints and Health-Related Quality of Life in Women With Breast Implants. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, 41, 661–668.
- Shoenfeld, Y.; Agmon-Levin, N. “ASIA”—Autoimmune/inflammatory syndrome induced by adjuvants. J. Autoimmun. 2011, 36, 4–8.
- Watad, A.; Rosenberg, V.; Tiosano, S.; Tervaert, J.W.C.; Yavne, Y.; Shoenfeld, Y.; Shalev, V.; Chodick, G.; Amital, H. Silicone breast implants and the risk of autoimmune/rheumatic disorders: A real-world analysis. Int. J. Epidemiol. 2018, 47, 1846–1854.
- Moraitis, S.D.; Agrafiotis, A.C.; Kapranou, A.; Kanakakis, K. Mediastinal silicone lymphadenopathy revealed after thymectomy for autoimmune myasthenia gravis. Monaldi Arch. Chest Dis. 2018, 88, 83–86.
- Barsky, A.J.; Borus, J.F. Functional somatic syndromes. Ann. Intern. Med. 1999, 130, 910–921.
- Vera-Lastra, O.; Medina, G.; Cruz-Dominguez, M.D.P.; Jara, L.J.; Shoenfeld, Y. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome): Clinical and immunological spectrum. Expert Rev. Clin. Immunol. 2013, 9, 361–373.
- Maijers, M.C.; de Blok, C.J.M.; Niessen, F.B.; van der Veldt, A.A.M.; Ritt, M.J.P.F.; Winters, H.A.H.; Kramer, M.H.H.; Nanayakkara, P.W.B. Women with silicone breast implants and unexplained systemic symptoms: A descriptive cohort study. Neth. J. Med. 2014, 71, 534–540.
- Magnusson, M.R.; Cooter, R.D.; Rakhorst, H.; McGuire, P.A.; Adams, W.P.; Deva, A.K. Breast Implant Illness: A Way Forward. Plast. Reconstr. Surg. 2019, 143, 74S–81S.
- Gadarowski, M.B.; Pukhalskaya, T.; Farah, R.; Smoller, B.R. Acquired anhidrosis in a patient with Sjogren syndrome and silicone breast implants. JAAD Case Rep. 2020, 6, 414–416.
- Akyol, L.; Onem, S.; Ozgen, M.; Sayarlioglu, M. Sjögren’s syndrome after silicone breast implantation. Eur. J. Rheumatol. 2015, 2, 165–166.
- Jara, L.J.; Medina, G.; Gómez-Bañuelos, E.; Saavedra, M.A.; Vera-Lastra, O. Still’s disease, lupus-like syndrome, and silicone breast implants. A case of “ASIA” (Shoenfeld’s syndrome). Lupus 2012, 21, 140–145.
- Lappe, M.A. Silicone-reactive disorder: A new autoimmune disease caused by immunostimulation and superantigens. Med. Hypotheses 1993, 41, 348–352.
- Tervaert, J.W.C.; Kappel, R.M. Silicone implant incompatibility syndrome (SIIS): A frequent cause of ASIA (Shoenfeld’s syndrome). Immunol. Res. 2013, 56, 293–298.
- Fuzzard, S.K.; Teixeira, R.; Zinn, R. A Review of the Literature on the Management of Silicone Implant Incompatibility Syndrome. Aesthet. Plast. Surg. 2019, 43, 1145–1149.
- Khoo, T.; Proudman, S.; Limaye, V. Silicone breast implants and depression, fibromyalgia and chronic fatigue syndrome in a rheumatology clinic population. Clin. Rheumatol. 2019, 38, 1271–1276.
- Colaris, M.J.L.; de Boer, M.; van der Hulst, R.R.; Tervaert, J.W.C. Two hundreds cases of ASIA syndrome following silicone implants: A comparative study of 30 years and a review of current literature. Immunol. Res. 2017, 65, 120–128.
- Felger, J.C.; Miller, A.H. Cytokine effects on the basal ganglia and dopamine function: The subcortical source of inflammatory malaise. Front. Neuroendocrinol. 2012, 33, 315–327.
- Tervaert, J.W.C.; Colaris, M.J.; van der Hulst, R.R. Silicone breast implants and autoimmune rheumatic diseases: Myth or reality. Curr. Opin. Rheumatol. 2017, 29, 348–354.
- de Faria Castro Fleury, E.; Rêgo, M.M.; Ramalho, L.C.; Ayres, V.J.; Seleti, R.O.; Ferreira, C.A.P.; Roveda, D., Jr. Silicone-induced granuloma of breast implant capsule (SIGBIC): Similarities and differences with anaplastic large cell lymphoma (ALCL) and their differential diagnosis. Breast Cancer Targets Ther. 2017, 9, 133–140.
- de Faria Castro Fleury, E.; D’Alessandro, G.S.; Wludarski, S.C.L. Silicone-induced granuloma of breast implant capsule (SIGBIC): Histopathology and radiological correlation. J. Immunol. Res. 2018, 2018, 6784971.
- Caravantes-Cortes, M.I.; Roldan-Valadez, E.; Zwojewski-Martinez, R.D.; Salazar-Ruiz, S.Y.; Carballo-Zarate, A.A. Breast Prosthesis Syndrome: Pathophysiology and Management Algorithm. Aesthet. Plast. Surg. 2020, 44, 1423–1437.
- Fleury, E.d.C.; Gianini, A.C.; Ayres, V.; Ramalho, L.C.; Seleti, R.O.; Roveda, D. Breast magnetic resonance imaging: Tips for the diagnosis of silicone-induced granuloma of a breast implant capsule (SIGBIC). Insights Imaging 2017, 8, 439–446.
- Favi, P.M.; Valencia, M.M.; Elliott, P.R.; Restrepo, A.; Gao, M.; Huang, H.; Pavon, J.J.; Webster, T.J. Shape and surface chemistry effects on the cytotoxicity and cellular uptake of metallic nanorods and nanospheres. J. Biomed. Mater. Res.—Part A 2015, 103, 3940–3955.
- Bhattacharjee, S.; de Haan, L.H.J.; Evers, N.M.; Jiang, X.; Marcelis, A.T.M.; Zuilhof, H.; Rietjens, I.M.C.M.; Alink, G.M. Role of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Part. Fibre Toxicol. 2010, 7, 25.
- Tervaert, J.W.C.; Mohazab, N.; Redmond, D.; van Eeden, C.; Osman, M. Breast implant illness: Scientific evidence of its existence. Expert Rev. Clin. Immunol. 2022, 18, 15–29.
- Katzin, W.E.; Centeno, J.A.; Feng, L.J.; Kiley, M.; Mullick, F.G. Pathology of lymph nodes from patients with breast implants: A histologic and spectroscopic evaluation. Am. J. Surg. Pathol. 2005, 29, 506–511.
- Ganau, S.; Tortajada, L.; Rodríguez, X.; González, G.; Sentís, M. Silicone lymphadenopathy: An unusual cause of internal mammary lymph node enlargement. Breast J. 2008, 14, 502–503.
- Soudack, M.; Yelin, A.; Simansky, D.; Ben-Nun, A. Fluorodeoxyglucose-positive internal mammary lymph node in breast cancer patients with silicone implants: Is it always metastatic cancer? Eur. J. Cardio-Thoracic Surg. 2013, 44, 79–82.
- Grubstein, A.; Cohen, M.; Steinmetz, A.; Cohen, D. Siliconomas mimicking cancer. Clin. Imaging 2011, 35, 228–231.
- Cohen Tervaert, J.W. Autoinflammatory/autoimmunity syndrome induced by adjuvants (ASIA; Shoenfeld’s syndrome): A new flame. Autoimmun. Rev. 2018, 17, 1259–1264.
- Karlson, E.W.; Lee, I.M.; Cook, N.R.; Manson, J.A.E.; Buring, J.E.; Hennekens, C.H. Comparison of self-reported diagnosis of connective tissue disease with medical records in female health professionals. The women’s health cohort study. Am. J. Epidemiol. 1999, 150, 652–660.
- Valente, D.S.; Zanella, R.K.; Mulazzani, C.M.; Valente, S.S. Risk Factors for Explantation of Breast Implants: A Cross-Sectional Study. Aesthet. Surg. J. 2021, 41, 923–928.
- Rohrich, R.J.; Kenkel, J.M.; Adams, W.P.; Beran, S.; Conner, W.C.H. A prospective analysis of patients undergoing silicone breast implant explantation. Plast. Reconstr. Surg. 2000, 105, 2529–2537.
- de Boer, M.; Colaris, M.; van der Hulst, R.R.W.J.; Tervaert, J.W.C. Is explantation of silicone breast implants useful in patients with complaints? Immunol. Res. 2017, 65, 25–36.
- Vasey, F.B.; Havice, D.L.; Bocanegra, T.S.; Seleznick, M.J.; Bridgeford, P.H.; Martinez-Osuna, P.; Espinoza, L.R. Clinical findings in symptomatic women with silicone breast implants. Semin. Arthritis Rheum. 1994, 24, 22–28.
- Aziz, N.; Vasey, F.; Leaverton, P. Comparison of clinical status among women retaining or removing gel breast implants. Am. J. Epidemiol. 1997, 145, 191.
- Thomas, W., 3rd; Harper, L.; Wong, S.; Michalski, J.; Harris, C.; Moore, J.; Rodning, C. Explantation of silicone breast implants. Am. Surg. 1997, 63, 421–429.
- Kappel, R.M.; Pruijn, G.J.M. The monobloc hydrogel breast implant, experiences and ideas. Eur. J. Plast. Surg. 2012, 35, 229–233.
- Svahn, J.; Vastine, V.; Landon, B.; Dobke, M. Outcome of mammary prostheses explantation: A patient perspective. Ann. Plast. Surg. 1996, 36, 594–600.
- Godfrey, P.; Godfrey, N. Response of locoregional and systemic symptoms to breast implant replacement with autologous tissues: Experience in 37 consecutive patients. Plast. Reconstr. Surg. 1996, 97, 110–116.
- Peters, W.; Smith, D.; Fornasier, V.; Lugowski, S.; Ibanez, D. An outcome analysis of 100 women after explantation of silicone gel breast implants. Ann. Plast. Surg. 1997, 39, 9–19.
- Hortolam, J.G.; de Carvalho, J.F.; Appenzeller, S. Connective tissue diseases following silicone breast implantation: Where do we stand? Clinics 2013, 68, 281.
- Singh, N.; Picha, G.J.; Hardas, B.; Schumacher, A.; Murphy, D.K. Five-year safety data for more than 55,000 subjects following breast implantation: Comparison of rare adverse event rates with silicone implants versus national norms and saline implants. Plast. Reconstr. Surg. 2017, 140, 666–679.
- Santiago, E.A.; de Paula, I.B. Autoimmune/Inflammatory Syndrome Induced by Adjuvants (Asia Syndrome) Associated with Silicone Breast Implant Rupture. Arch. Breast Cancer 2021, 8, 156–161.
- Jara, L.J.; García-Collinot, G.; Medina, G.; Cruz-Dominguez, M.d.; Vera-Lastra, O.; Carranza-Muleiro, R.A.; Saavedra, M.A. Severe manifestations of autoimmune syndrome induced by adjuvants (Shoenfeld’s syndrome). Immunol. Res. 2017, 65, 8–16.
- Barbosa, M.R.; Makris, U.E.; Mansi, I.A. Association of Breast Implants with Nonspecific Symptoms, Connective Tissue Diseases, and Allergic Reactions: A Retrospective Cohort Analysis. Plast. Reconstr. Surg. 2021, 147, 42E–49E.
This entry is offline, you can click here to edit this entry!
