Peritoneal carcinomatosis (PC) is a poor prognostic factor for all malignancies. This extent of metastatic disease progression remains difficult to treat with systemic therapies due to poor peritoneal vascularization resulting in limited drug delivery and penetration into tissues. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) are surgical interventions that directly target peritoneal tumors and have improved outcomes for PC resulting from appendiceal and colorectal cancer (CRC). Despite these radical therapies, long-term survival remains infrequent, and recurrence is common. The reasons for these outcomes are multifactorial and signal the need for the continued development of novel therapeutics, techniques, and approaches to improve outcomes for these patients.
- cytoreductive surgery (CRS)
- hyperthermic intraperitoneal chemotherapy (HIPEC)
- peritoneal surface malignancies (PSM)
- colon cancer
- appendiceal cancer
- peritoneal carcinomatosis
1. Introduction
2. Background
2.1. The Peritoneum
2.2. Clinical Presentation of PC
2.3. CRS and HIPEC Beginnings
3. Prognostic Indicators
3.1. Preoperative Patient Selection
3.1.1. Prior Surgical Score (PSS)
3.1.2. Peritoneal Carcinomatosis Index (PCI)
3.1.3. Peritoneal Surface Disease Severity Score (PSDSS)
3.2. Predicting Postoperative Outcomes
3.2.1. Completeness of Cytoreduction (CC) Score
3.2.2. Resection (R) Score
3.3. Emerging Predictive Modalities
4. HIPEC Techniques
4.1. Open HIPEC
4.2. Closed HIPEC
4.3. Laparoscopic HIPEC
5. HIPEC Safety
6. Landmark Efficacy Studies
6.1. HIPEC with Curative Intent
| Primary Malignancy |
Agent | Duration | Dose/ Concentration |
Bidirectional IV Therapy | Ref. |
|---|---|---|---|---|---|
| Appendiceal | MMC | 90 min | 30 mg (0 min) + 10 mg (60 min) | -- | [116][117] |
| 90–120 min | 30 mg/m2 | -- | [116][117] | ||
| MMC + doxorubicin | 90 min | 15 mg/m2, 15 mg/m2 | 5-FU (400 mg/m2) + Leucovorin (20 mg/m2) |
[116][117] | |
| Oxaliplatin | 30 min | 300 mg/m2 | 5-FU (400 mg/m2) + Leucovorin (20 mg/m2) |
[116] | |
| Colorectal | MMC | 90 min | 30 mg (0 min) + 10 mg (60 min) | -- | [117][118][119] |
| 90–110 min | 30 mg/m2 | -- | [117][118][119] | ||
| Oxaliplatin (only with PCI 11–15) |
30 min | 300 mg/m2 | 5-FU (400 mg/m2) + Leucovorin (20 mg/m2) |
[118] |
6.2. HIPEC for Palliation
6.3. HIPEC for Prevention of Disease Recurrence
6.4. Alternative Intraperitoneal Chemotherapy Techniques
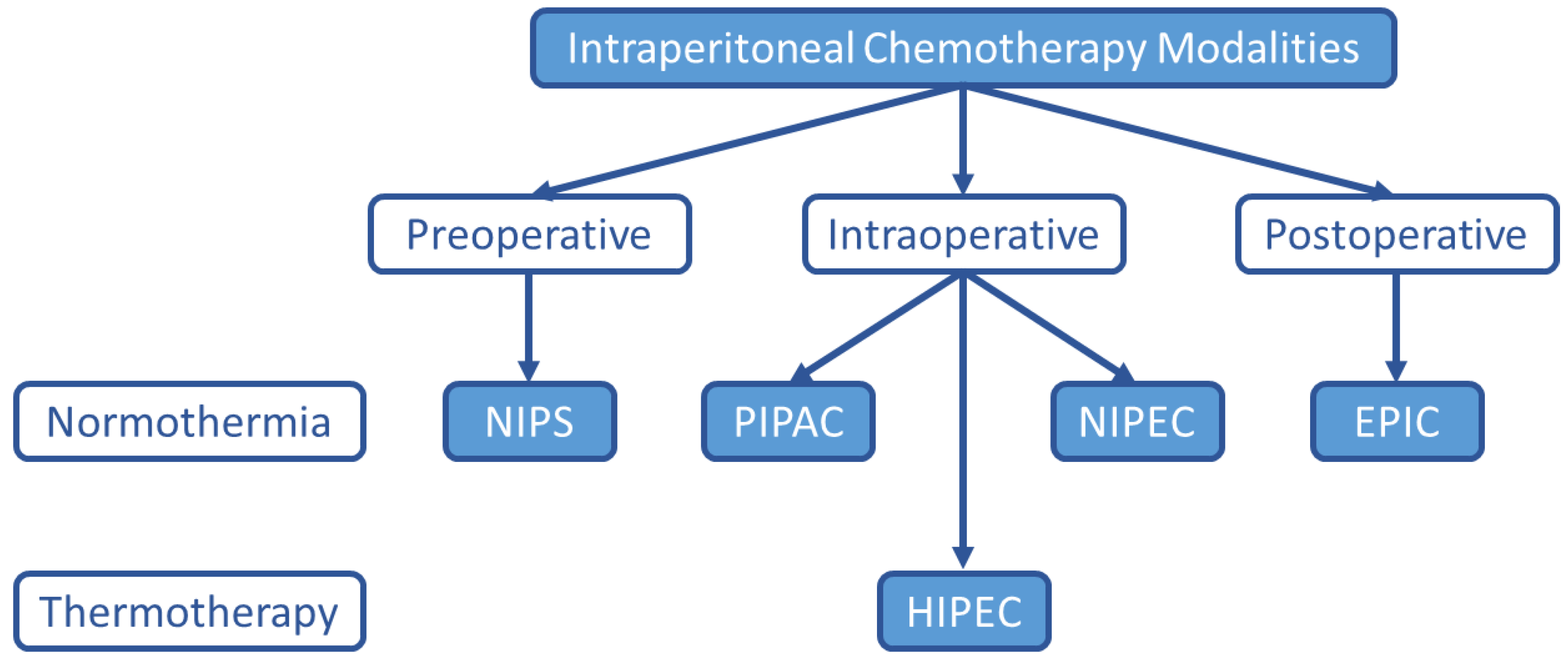
6.4.1. Early Postoperative Intraperitoneal Chemotherapy (EPIC)
6.4.2. Normothermic Intraperitoneal Chemotherapy (NIPEC)
6.4.3. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC)
6.4.4. Neoadjuvant Intraperitoneal and Systemic Chemotherapy (NIPS)
6.5. HIPEC Agents and Adjunctive Therapies
6.5.1. Current Regimens
6.5.2. Emerging Therapeutics
This entry is adapted from the peer-reviewed paper 10.3390/jcm11102840
References
- Jafari, M.D.; Halabi, W.J.; Stamos, M.J.; Nguyen, V.Q.; Carmichael, J.C.; Mills, S.D.; Pigazzi, A. Surgical outcomes of hyperthermic intraperitoneal chemotherapy: Analysis of the american college of surgeons national surgical quality improvement program. JAMA Surg. 2014, 149, 170–175.
- Pletcher, E.; Gleeson, E.; Labow, D. Peritoneal Cancers and Hyperthermic Intraperitoneal Chemotherapy. Surg. Clin. N. Am. 2020, 100, 589–613.
- Auer, R.C.; Sivajohanathan, D.; Biagi, J.; Conner, J.; Kennedy, E.; May, T. Indications for hyperthermic intraperitoneal chemotherapy with cytoreductive surgery: A systematic review. Eur. J. Cancer 2020, 127, 76–95.
- Flanagan, M.; Solon, J.; Chang, K.H.; Deady, S.; Moran, B.; Cahill, R.; Shields, C.; Mulsow, J. Peritoneal metastases from extra-abdominal cancer—A population-based study. Eur. J. Surg. Oncol. 2018, 44, 1811–1817.
- Nadler, A.; McCart, J.A.; Govindarajan, A. Peritoneal Carcinomatosis from Colon Cancer: A Systematic Review of the Data for Cytoreduction and Intraperitoneal Chemotherapy. Clin. Colon. Rectal. Surg. 2015, 28, 234–246.
- McCusker, M.E.; Cote, T.R.; Clegg, L.X.; Sobin, L.H. Primary malignant neoplasms of the appendix: A population-based study from the surveillance, epidemiology and end-results program, 1973–1998. Cancer 2002, 94, 3307–3312.
- De Bree, E.; Koops, W.; Kröger, R.; van Ruth, S.; Witkamp, A.J.; Zoetmulder, F.A. Peritoneal carcinomatosis from colorectal or appendiceal origin: Correlation of preoperative CT with intraoperative findings and evaluation of interobserver agreement. J. Surg. Oncol. 2004, 86, 64–73.
- Koppe, M.J.; Boerman, O.C.; Oyen, W.J.G.; Bleichrodt, R.P. Peritoneal Carcinomatosis of Colorectal Origin: Incidence and Current Treatment Strategies. Ann. Surg. 2006, 243, 212.
- McQuellon, R.P.; Loggie, B.W.; Lehman, A.B.; Russell, G.B.; Fleming, R.A.; Shen, P.; Levine, E.A. Long-term survivorship and quality of life after cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann. Surg. Oncol. 2003, 10, 155–162.
- Maciver, A.H.; Al-Sukhni, E.; Esquivel, J.; Skitzki, J.J.; Kane, J.M., 3rd; Francescutti, V.A. Current Delivery of Hyperthermic Intraperitoneal Chemotherapy with Cytoreductive Surgery (CS/HIPEC) and Perioperative Practices: An International Survey of High-Volume Surgeons. Ann. Surg. Oncol. 2017, 24, 923–930.
- Bushati, M.; Rovers, K.P.; Sommariva, A.; Sugarbaker, P.H.; Morris, D.L.; Yonemura, Y.; Quadros, C.A.; Somashekhar, S.P.; Ceelen, W.; Dubé, P.; et al. The current practice of cytoreductive surgery and HIPEC for colorectal peritoneal metastases: Results of a worldwide web-based survey of the Peritoneal Surface Oncology Group International (PSOGI). Eur. J. Surg. Oncol. 2018, 44, 1942–1948.
- Isaza-Restrepo, A.; Martin-Saavedra, J.S.; Velez-Leal, J.L.; Vargas-Barato, F.; Riveros-Dueñas, R. The Peritoneum: Beyond the Tissue—A Review. Front. Physiol. 2018, 9, 738.
- Solass, W.; Horvath, P.; Struller, F.; Königsrainer, I.; Beckert, S.; Königsrainer, A.; Weinreich, F.J.; Schenk, M. Functional vascular anatomy of the peritoneum in health and disease. Pleura Peritoneum 2016, 1, 145–158.
- Van Baal, J.O.; Van de Vijver, K.K.; Nieuwland, R.; van Noorden, C.J.; van Driel, W.J.; Sturk, A.; Kenter, G.G.; Rikkert, L.G.; Lok, C.A. The histophysiology and pathophysiology of the peritoneum. Tissue Cell 2017, 49, 95–105.
- Lemoine, L.; Sugarbaker, P.; Van der Speeten, K. Pathophysiology of colorectal peritoneal carcinomatosis: Role of the peritoneum. World J. Gastroenterol. 2016, 22, 7692–7707.
- Giorgio, A.; Pinto, E. Treatment of Peritoneal Surface Malignancies: State of the Art and Perspectives; Springer: Milano, Italy, 2015.
- Terzi, C.; Arslan, N.C.; Canda, A.E. Peritoneal carcinomatosis of gastrointestinal tumors: Where are we now? World J. Gastroenterol. 2014, 20, 14371–14380.
- Miller, J.; Wynn, W.H. A malignant tumour arising from the endothelium of the peritoneum, and producing a mucoid ascitic fluid. J. Pathol. Bacteriol. 1908, 12, 267–278.
- Chu, D.Z.; Lang, N.P.; Thompson, C.; Osteen, P.K.; Westbrook, K.C. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer 1989, 63, 364–367.
- De Cuba, E.M.; Kwakman, R.; Knol, D.L.; Bonjer, H.J.; Meijer, G.A.; Te Velde, E.A. Cytoreductive surgery and HIPEC for peritoneal metastases combined with curative treatment of colorectal liver metastases: Systematic review of all literature and meta-analysis of observational studies. Cancer Treat. Rev. 2013, 39, 321–327.
- Seifert, P.; Baker, L.H.; Reed, M.L.; Vaitkevicius, V.K. Comparison of continuously infused 5-fluorouracil with bolus injection in treatment of patients with colorectal adenocarcinoma. Cancer 1975, 36, 123–128.
- Esquivel, J. Advances in the Management of Peritoneal Carcinomatosis; Future Medicine Ltd.: London, UK, 2014.
- Meigs, J.V. Tumors of the Female Pelvic Organs; The Macmillan Company: New York, NY, USA, 1934.
- Goodman, M.D.; McPartland, S.; Detelich, D.; Saif, M.W. Chemotherapy for intraperitoneal use: A review of hyperthermic intraperitoneal chemotherapy and early post-operative intraperitoneal chemotherapy. J. Gastrointest. Oncol. 2016, 7, 45–57.
- Dedrick, R.L.; Myers, C.E.; Bungay, P.M.; DeVita, V.T., Jr. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat. Rep. 1978, 62, 1–13.
- Larkin, J.M.; Edwards, W.S.; Smith, D.E.; Clark, P.J. Systemic thermotherapy: Description of a method and physiologic tolerance in clinical subjects. Cancer 1977, 40, 3155–3159.
- Long, R.T.; Spratt, J.S., Jr.; Dowling, E. Pseudomyxoma peritonei. New concepts in management with a report of seventeen patients. Am. J. Surg. 1969, 117, 162–169.
- Spratt, J.S.; Adcock, R.A.; Muskovin, M.; Sherrill, W.; McKeown, J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980, 40, 256–260.
- Shingleton, W.W.; Parker, R.T. Abdominal perfusion for cancer chemotherapy using hypothermia and hyperthermia. Acta Unio Int. Contra Cancrum 1964, 20, 465–468.
- Fujimoto, S.; Shrestha, R.D.; Kokubun, M.; Ohta, M.; Takahashi, M.; Kobayashi, K.; Kiuchi, S.; Okui, K.; Miyoshi, T.; Arimizu, N.; et al. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann. Surg. 1988, 208, 36–41.
- Sugarbaker, P.H. Peritonectomy procedures. Ann. Surg. 1995, 221, 29–42.
- Bhatt, A.; Glehen, O. Extent of Peritoneal Resection for Peritoneal Metastases: Looking Beyond a Complete Cytoreduction. Ann. Surg. Oncol. 2020, 27, 1458–1470.
- Bhatt, A.; Yonemura, Y.; Mehta, S.; Benzerdjeb, N.; Kammar, P.; Parikh, L.; Shah, M.Y.; Shaikh, S.; Prabhu, A.; Mishra, S.; et al. Target region resection in patients undergoing cytoreductive surgery for peritoneal metastases-is it necessary in absence of visible disease? Eur. J. Surg. Oncol. 2020, 46, 582–589.
- Glehen, O.; Kwiatkowski, F.; Sugarbaker, P.H.; Elias, D.; Levine, E.A.; De Simone, M.; Barone, R.; Yonemura, Y.; Cavaliere, F.; Quenet, F.; et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J. Clin. Oncol. 2004, 22, 3284–3292.
- Glehen, O.; Gilly, F.N.; Boutitie, F.; Bereder, J.M.; Quenet, F.; Sideris, L.; Mansvelt, B.; Lorimier, G.; Msika, S.; Elias, D. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Cancer 2010, 116, 5608–5618.
- Rosa, F.; Galiandro, F.; Ricci, R.; Di Miceli, D.; Quero, G.; Fiorillo, C.; Cina, C.; Alfieri, S. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal peritoneal metastases: Analysis of short- and long-term outcomes. Langenbecks Arch. Surg. 2021.
- Yu, Y.; Li, X.B.; Lin, Y.L.; Ma, R.; Ji, Z.H.; Zhang, Y.B.; An, S.L.; Liu, G.; Yang, X.J.; Li, Y. Efficacy of 1 384 cases of peritoneal carcinomatosis underwent cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. Zhonghua Wei Chang Wai Ke Za Zhi 2021, 24, 230–239.
- Grotz, T.E.; Overman, M.J.; Eng, C.; Raghav, K.P.; Royal, R.E.; Mansfield, P.F.; Mann, G.N.; Robinson, K.A.; Beaty, K.A.; Rafeeq, S.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Moderately and Poorly Differentiated Appendiceal Adenocarcinoma: Survival Outcomes and Patient Selection. Ann. Surg. Oncol. 2017, 24, 2646–2654.
- Elias, D.; Lefevre, J.H.; Chevalier, J.; Brouquet, A.; Marchal, F.; Classe, J.M.; Ferron, G.; Guilloit, J.M.; Meeus, P.; Goéré, D.; et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J. Clin. Oncol. 2009, 27, 681–685.
- Sugarbaker, P.H. Preoperative Assessment of Cancer Patients with Peritoneal Metastases for Complete Cytoreduction. Indian J. Surg. Oncol. 2016, 7, 295–302.
- Ashvin, R.; Aditi, B.; Nikhilesh, J. Preoperative Management of Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Indian J. Surg. Oncol. 2017, 8, 573–579.
- Milovanov, V.; Sardi, A.; Aydin, N.; Nieroda, C.; Sittig, M.; Nunez, M.; Gushchin, V. Extensive surgical history prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy is associated with poor survival outcomes in patients with peritoneal mucinous carcinomatosis of appendiceal origin. Eur. J. Surg. Oncol. 2015, 41, 881–885.
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374.
- Sugarbaker, P.H.; Jablonski, K.A. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann. Surg. 1995, 221, 124–132.
- Lee, R.M.; Zaidi, M.Y.; Gamboa, A.C.; Speegle, S.; Kimbrough, C.W.; Cloyd, J.M.; Leiting, J.L.; Grotz, T.E.; Lee, A.J.; Fournier, K.F.; et al. What is the Optimal Preoperative Imaging Modality for Assessing Peritoneal Cancer Index? An Analysis From the United States HIPEC Collaborative. Clin. Colorectal Cancer 2020, 19, e1–e7.
- Iversen, L.H.; Rasmussen, P.C.; Laurberg, S. Value of laparoscopy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Br. J. Surg. 2013, 100, 285–292.
- Marmor, R.A.; Kelly, K.J.; Lowy, A.M.; Baumgartner, J.M. Laparoscopy is Safe and Accurate to Evaluate Peritoneal Surface Metastasis Prior to Cytoreductive Surgery. Ann. Surg. Oncol. 2016, 23, 1461–1467.
- Harmon, R.L.; Sugarbaker, P.H. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int. Semin. Surg. Oncol. 2005, 2, 3.
- Mehta, S.S.; Bhatt, A.; Glehen, O. Cytoreductive Surgery and Peritonectomy Procedures. Indian J. Surg. Oncol. 2016, 7, 139–151.
- Goéré, D.; Souadka, A.; Faron, M.; Cloutier, A.S.; Viana, B.; Honoré, C.; Dumont, F.; Elias, D. Extent of colorectal peritoneal carcinomatosis: Attempt to define a threshold above which HIPEC does not offer survival benefit: A comparative study. Ann. Surg. Oncol. 2015, 22, 2958–2964.
- Chia, C.S.; Seshadri, R.A.; Kepenekian, V.; Vaudoyer, D.; Passot, G.; Glehen, O. Survival outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from gastric cancer: A systematic review. Pleura Peritoneum 2016, 1, 67–77.
- Sugarbaker, P.H. Management of Peritoneal Surface Malignancy Using Intraperitoneal Chemotherapy and Cytoreductive Surgery: Manual for Physicians and Nurses; Ludann Company: Lowell, MI, USA, 1998.
- Esquivel, J.; Lowy, A.M.; Markman, M.; Chua, T.; Pelz, J.; Baratti, D.; Baumgartner, J.M.; Berri, R.; Bretcha-Boix, P.; Deraco, M.; et al. The American Society of Peritoneal Surface Malignancies (ASPSM) Multiinstitution Evaluation of the Peritoneal Surface Disease Severity Score (PSDSS) in 1,013 Patients with Colorectal Cancer with Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2014, 21, 4195–4201.
- Ng, J.L.; Ong, W.S.; Chia, C.S.; Tan, G.H.; Soo, K.C.; Teo, M.C. Prognostic Relevance of the Peritoneal Surface Disease Severity Score Compared to the Peritoneal Cancer Index for Colorectal Peritoneal Carcinomatosis. Int. J. Surg. Oncol. 2016, 2016, 2495131.
- Yoon, W.; Alame, A.; Berri, R. Peritoneal Surface Disease Severity Score as a predictor of resectability in the treatment of peritoneal surface malignancies. Am. J. Surg. 2014, 207, 403–407; discussion 406–407.
- Esquivel, J.; Garcia, S.S.; Hicken, W.; Seibel, J.; Shekitka, K.; Trout, R. Evaluation of a new staging classification and a Peritoneal Surface Disease Severity Score (PSDSS) in 229 patients with mucinous appendiceal neoplasms with or without peritoneal dissemination. J. Surg. Oncol. 2014, 110, 656–660.
- Sugarbaker, P.H. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother. Pharmacol. 1999, 43, S15–S25.
- Chua, T.C.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Glehen, O.; Gilly, F.N.; Baratti, D.; Deraco, M.; Elias, D.; Sardi, A.; et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Clin. Oncol. 2012, 30, 2449–2456.
- Chua, T.C.; Yan, T.D.; Smigielski, M.E.; Zhu, K.J.; Ng, K.M.; Zhao, J.; Morris, D.L. Long-term survival in patients with pseudomyxoma peritonei treated with cytoreductive surgery and perioperative intraperitoneal chemotherapy: 10 years of experience from a single institution. Ann. Surg. Oncol. 2009, 16, 1903–1911.
- Munoz-Zuluaga, C.A.; King, M.C.; Diaz-Sarmiento, V.S.; Studeman, K.; Sittig, M.; MacDonald, R.; Nieroda, C.; Zambrano-Vera, K.; Gushchin, V.; Sardi, A. Defining “Complete Cytoreduction” After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (CRS/HIPEC) for the Histopathologic Spectrum of Appendiceal Carcinomatosis. Ann. Surg. Oncol. 2020, 27, 5026–5036.
- Aziz, O.; Jaradat, I.; Chakrabarty, B.; Selvasekar, C.R.; Fulford, P.E.; Saunders, M.P.; Renehan, A.G.; Wilson, M.S.; O’Dwyer, S.T. Predicting Survival After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Appendix Adenocarcinoma. Dis. Colon Rectum 2018, 61, 795–802.
- Levine, E.A.; Stewart, J.H.T.; Shen, P.; Russell, G.B.; Loggie, B.L.; Votanopoulos, K.I. Intraperitoneal chemotherapy for peritoneal surface malignancy: Experience with 1000 patients. J. Am. Coll. Surg. 2014, 218, 573–585.
- Levine, E.A.; Stewart, J.H.T.; Russell, G.B.; Geisinger, K.R.; Loggie, B.L.; Shen, P. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: Experience with 501 procedures. J. Am. Coll. Surg. 2007, 204, 943–953; discussion 945–953.
- Elias, D.; Glehen, O.; Pocard, M.; Quenet, F.; Goéré, D.; Arvieux, C.; Rat, P.; Gilly, F. A comparative study of complete cytoreductive surgery plus intraperitoneal chemotherapy to treat peritoneal dissemination from colon, rectum, small bowel, and nonpseudomyxoma appendix. Ann. Surg. 2010, 251, 896–901.
- Beagan, J.J.; Sluiter, N.R.; Bach, S.; Eijk, P.P.; Vlek, S.L.; Heideman, D.A.M.; Kusters, M.; Pegtel, D.M.; Kazemier, G.; van Grieken, N.C.T.; et al. Circulating Tumor DNA as a Preoperative Marker of Recurrence in Patients with Peritoneal Metastases of Colorectal Cancer: A Clinical Feasibility Study. J. Clin. Med. 2020, 9, 1738.
- Melero, J.T.; Ortega, F.G.; Gonzalez, A.M.; Carmona-Saez, P.; Garcia Puche, J.L.; Sugarbaker, P.H.; Delgado, M.; Lorente, J.A.; Serrano, M.J. Prognostic factor analysis of circulating tumor cells in peripheral blood of patients with peritoneal carcinomatosis of colon cancer origin treated with cytoreductive surgery plus an intraoperative hyperthermic intraperitoneal chemotherapy procedure (CRS + HIPEC). Surgery 2016, 159, 728–735.
- Kusamura, S.; Hutanu, I.; Baratti, D.; Deraco, M. Circulating tumor markers: Predictors of incomplete cytoreduction and powerful determinants of outcome in pseudomyxoma peritonei. J. Surg. Oncol. 2013, 108, 1–8.
- Garland-Kledzik, M.; Uppal, A.; Naeini, Y.B.; Stern, S.; Erali, R.; Scholer, A.J.; Khader, A.M.; Santamaria-Barria, J.A.; Cummins-Perry, K.; Zhou, Y.; et al. Prognostic Impact and Utility of Immunoprofiling in the Selection of Patients with Colorectal Peritoneal Carcinomatosis for Cytoreductive Surgery (CRS) and Heated Intraperitoneal Chemotherapy (HIPEC). J. Gastrointest. Surg. 2021, 25, 233–240.
- Seretis, F.; Seretis, C. Immuno-PCI: A proposal for the implementation of "seed and soil" concept in the treatment of peritoneal carcinomatosis from colorectal cancer. Med. Hypotheses 2014, 83, 137–141.
- Simkens, G.A.; Wintjens, A.; Rovers, K.P.; Nienhuijs, S.W.; de Hingh, I.H. Effective Strategies to Predict Survival of Colorectal Peritoneal Metastases Patients Eligible for Cytoreductive Surgery and HIPEC. Cancer Manag. Res. 2021, 13, 5239–5249.
- Kyriazanos, I.; Kalles, V.; Stefanopoulos, A.; Spiliotis, J.; Mohamed, F. Operating personnel safety during the administration of Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Surg. Oncol. 2016, 25, 308–314.
- Leiting, J.L.; Cloyd, J.M.; Ahmed, A.; Fournier, K.; Lee, A.J.; Dessureault, S.; Felder, S.; Veerapong, J.; Baumgartner, J.M.; Clarke, C.; et al. Comparison of open and closed hyperthermic intraperitoneal chemotherapy: Results from the United States hyperthermic intraperitoneal chemotherapy collaborative. World J. Gastrointest. Oncol. 2020, 12, 756–767.
- Arjona-Sanchez, A.; Esquivel, J.; Glehen, O.; Passot, G.; Turaga, K.K.; Labow, D.; Rufian-Peña, S.; Morales, R.; van der Speeten, K. A minimally invasive approach for peritonectomy procedures and hyperthermic intraperitoneal chemotherapy (HIPEC) in limited peritoneal carcinomatosis: The American Society of Peritoneal Surface Malignancies (ASPSM) multi-institution analysis. Surg. Endosc. 2019, 33, 854–860.
- Arjona-Sanchez, A.; Aziz, O.; Passot, G.; Salti, G.; Esquivel, J.; Van der Speeten, K.; Piso, P.; Nedelcut, D.S.; Sommariva, A.; Yonemura, Y.; et al. Laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for limited peritoneal metastasis. The PSOGI international collaborative registry. Eur. J. Surg. Oncol. 2021, 47, 1420–1426.
- Passot, G.; Bakrin, N.; Isaac, S.; Decullier, E.; Gilly, F.N.; Glehen, O.; Cotte, E. Postoperative outcomes of laparoscopic vs open cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for treatment of peritoneal surface malignancies. Eur. J. Surg. Oncol. 2014, 40, 957–962.
- Halkia, E.A.; Kyriazanos, J.; Efstathiou, E.; Spiliotis, J.D. Laparoscopic hyperthermic intraperitoneal chemotherapy for the management of advanced peritoneal carcinomatosis. Hepatogastroenterology 2011, 58, 1915–1917.
- Facchiano, E.; Risio, D.; Kianmanesh, R.; Msika, S. Laparoscopic hyperthermic intraperitoneal chemotherapy: Indications, aims, and results: A systematic review of the literature. Ann. Surg. Oncol. 2012, 19, 2946–2950.
- Sommariva, A.; Zagonel, V.; Rossi, C.R. The role of laparoscopy in peritoneal surface malignancies selected for hyperthermic intraperitoneal chemotherapy (HIPEC). Ann. Surg. Oncol. 2012, 19, 3737–3744.
- Klaver, C.E.L.; Stam, R.; Sloothaak, D.A.M.; Crezee, J.; Bemelman, W.A.; Punt, C.J.A.; Tanis, P.J. Colorectal cancer at high risk of peritoneal metastases: Long term outcomes of a pilot study on adjuvant laparoscopic HIPEC and future perspectives. Oncotarget 2017, 8, 51200–51209.
- Abudeeb, H.; Selvasekar, C.R.; O’Dwyer, S.T.; Chakrabarty, B.; Malcolmson, L.; Renehan, A.G.; Wilson, M.S.; Aziz, O. Laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for perforated low-grade appendiceal mucinous neoplasms. Surg. Endosc. 2020, 34, 5516–5521.
- Arjona-Sánchez, A.; Cortés-Guiral, D.; Duran-Martínez, M.; Villarejo-Campos, P.; Sánchez-Hidalgo, J.M.; Casado-Adam, A.; Rodriguez-Ortiz, L.; Romero-Ruiz, A.; Rufian-Andujar, B.; Espinosa-Redondo, E.; et al. Complete laparoscopic pelvic peritonectomy plus hyperthermic intraperitoneal chemotherapy. Tech. Coloproctol. 2020, 24, 1083–1088.
- Blumenthaler, A.N.; Allen, C.J.; Ikoma, N.; Blum, M.; Das, P.; Minsky, B.D.; Mansfield, P.F.; Ajani, J.A.; Badgwell, B.D. Laparoscopic HIPEC for Low-Volume Peritoneal Metastasis in Gastric and Gastroesophageal Adenocarcinoma. Ann. Surg. Oncol. 2020, 27, 5047–5056.
- Mercier, F.; Jeremie, G.; Alyami, M.; Delphine, V.; Vahan, K.; Pascal, R.; Sylvie, I.; Guillaume, P.; Olivier, G. Long-term results of laparoscopic cytoreductive surgery and HIPEC for the curative treatment of low-grade pseudomyxoma peritonei and multicystic mesothelioma. Surg. Endosc. 2020, 34, 4916–4923.
- Rodríguez-Ortiz, L.; Arjona-Sánchez, A.; Ibañez-Rubio, M.; Sánchez-Hidalgo, J.; Casado-Adam, A.; Rufián-Peña, S.; Briceño-Delgado, J. Laparoscopic cytoreductive surgery and HIPEC: A comparative matched analysis. Surg. Endosc. 2021, 35, 1778–1785.
- Cusumano, C.; Carrere, S.; Bouillin, A.; Nougaret, S.; Khellaf, L.; Quénet, F.; Sgarbura, O. Laparoscopic cytoreductive surgery and HIPEC in LAMN with small volume of peritoneal disease: A valuable option of treatment for good patient-related experience measures (PREMs). Surg. Endosc. 2021.
- Chua, T.C.; Yan, T.D.; Saxena, A.; Morris, D.L. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: A systematic review of morbidity and mortality. Ann. Surg. 2009, 249, 900–907.
- Desantis, M.; Bernard, J.L.; Casanova, V.; Cegarra-Escolano, M.; Benizri, E.; Rahili, A.M.; Benchimol, D.; Bereder, J.M. Morbidity, mortality, and oncological outcomes of 401 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC). Langenbecks Arch. Surg. 2015, 400, 37–48.
- Foster, J.M.; Sleightholm, R.; Patel, A.; Shostrom, V.; Hall, B.; Neilsen, B.; Bartlett, D.; Smith, L. Morbidity and Mortality Rates Following Cytoreductive Surgery Combined With Hyperthermic Intraperitoneal Chemotherapy Compared With Other High-Risk Surgical Oncology Procedures. JAMA Netw. Open 2019, 2, e186847.
- Dodson, R.M.; McQuellon, R.P.; Mogal, H.D.; Duckworth, K.E.; Russell, G.B.; Votanopoulos, K.I.; Shen, P.; Levine, E.A. Quality-of-Life Evaluation after Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2016, 23, 772–783.
- Polanco, P.M.; Ding, Y.; Knox, J.M.; Ramalingam, L.; Jones, H.; Hogg, M.E.; Zureikat, A.H.; Holtzman, M.P.; Pingpank, J.; Ahrendt, S.; et al. Institutional Learning Curve of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemoperfusion for Peritoneal Malignancies. Ann. Surg. Oncol. 2015, 22, 1673–1679.
- Kusamura, S.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Elias, D.; Baratti, D.; Morris, D.L.; Sardi, A.; Glehen, O.; Deraco, M. Multicentre study of the learning curve and surgical performance of cytoreductive surgery with intraperitoneal chemotherapy for pseudomyxoma peritonei. Br. J. Surg. 2014, 101, 1758–1765.
- Benson, A.B., 3rd; Venook, A.P.; Bekaii-Saab, T.; Chan, E.; Chen, Y.J.; Cooper, H.S.; Engstrom, P.F.; Enzinger, P.C.; Fenton, M.J.; Fuchs, C.S.; et al. Colon cancer, version 3.2014. J. Natl. Compr. Canc. Netw. 2014, 12, 1028–1059.
- Benson, A.B., 3rd; Venook, A.P.; Cederquist, L.; Chan, E.; Chen, Y.J.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; Enzinger, P.C.; Fichera, A.; et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2017, 15, 370–398.
- The Chicago Consensus on peritoneal surface malignancies: Standards. Cancer 2020, 126, 2516–2524.
- Rajeev, R.; Klooster, B.; Turaga, K.K. Impact of surgical volume of centers on post-operative outcomes from cytoreductive surgery and hyperthermic intra-peritoneal chemoperfusion. J. Gastrointest. Oncol. 2016, 7, 122–128.
- Schuitevoerder, D.; Sherman, S.K.; Izquierdo, F.J.; Eng, O.S.; Turaga, K.K. Assessment of the Surgical Workforce Pertaining to Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in the United States. Ann. Surg. Oncol. 2020, 27, 3097–3102.
- Andréasson, H.; Lorant, T.; Påhlman, L.; Graf, W.; Mahteme, H. Cytoreductive surgery plus perioperative intraperitoneal chemotherapy in pseudomyxoma peritonei: Aspects of the learning curve. Eur. J. Surg. Oncol. 2014, 40, 930–936.
- Rog, C.J.; Lucas, G.; Reiter, S.; Vetto, S.; Alassas, M.; Ong, E.S. Optimal oncologic and perioperative outcomes of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy are attainable at a community center. Am. J. Surg. 2021, 221, 1200–1202.
- Schmidt, C.; Creutzenberg, M.; Piso, P.; Hobbhahn, J.; Bucher, M. Peri-operative anaesthetic management of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Anaesthesia 2008, 63, 389–395.
- Nizri, E.; Kusamura, S.; Fallabrino, G.; Guaglio, M.; Baratti, D.; Deraco, M. Dose-Dependent Effect of Red Blood Cells Transfusion on Perioperative and Long-Term Outcomes in Peritoneal Surface Malignancies Treated with Cytoreduction and HIPEC. Ann. Surg. Oncol. 2018, 25, 3264–3270.
- Yan, T.D.; Links, M.; Fransi, S.; Jacques, T.; Black, D.; Saunders, V.; Morris, D.L. Learning curve for cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal surface malignancy--a journey to becoming a Nationally Funded Peritonectomy Center. Ann. Surg. Oncol. 2007, 14, 2270–2280.
- Kubi, B.; Nudotor, R.; Fackche, N.; Nizam, W.; Cloyd, J.M.; Grotz, T.E.; Fournier, K.F.; Dineen, S.P.; Powers, B.D.; Veerapong, J.; et al. Impact of Perioperative Blood Transfusions on Outcomes After Hyperthermic Intraperitoneal Chemotherapy: A Propensity-Matched Analysis. Ann. Surg. Oncol. 2021, 28, 4499–4507.
- Saxena, A.; Yan, T.D.; Chua, T.C.; Fransi, S.; Almohaimeed, K.; Ahmed, S.; Morris, D.L. Risk factors for massive blood transfusion in cytoreductive surgery: A multivariate analysis of 243 procedures. Ann. Surg. Oncol. 2009, 16, 2195–2203.
- Saxena, A.; Valle, S.J.; Liauw, W.; Morris, D.L. Allogenic Blood Transfusion Is an Independent Predictor of Poorer Peri-operative Outcomes and Reduced Long-Term Survival after Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: A Review of 936 Cases. J. Gastrointest. Surg. 2017, 21, 1318–1327.
- Miki, C.; Hiro, J.; Ojima, E.; Inoue, Y.; Mohri, Y.; Kusunoki, M. Perioperative allogeneic blood transfusion, the related cytokine response and long-term survival after potentially curative resection of colorectal cancer. Clin. Oncol. 2006, 18, 60–66.
- Sargant, N.; Roy, A.; Simpson, S.; Chandrakumaran, K.; Alves, S.; Coakes, J.; Bell, J.; Knight, J.; Wilson, P.; Mohamed, F.; et al. A protocol for management of blood loss in surgical treatment of peritoneal malignancy by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Transfus. Med. 2016, 26, 118–122.
- Saxena, A.; Chua, T.C.; Fransi, S.; Liauw, W.; Morris, D.L. Effectiveness of early and aggressive administration of fresh frozen plasma to reduce massive blood transfusion during cytoreductive surgery. J. Gastrointest. Oncol. 2013, 4, 30–39.
- Musallam, K.M.; Tamim, H.M.; Richards, T.; Spahn, D.R.; Rosendaal, F.R.; Habbal, A.; Khreiss, M.; Dahdaleh, F.S.; Khavandi, K.; Sfeir, P.M.; et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: A retrospective cohort study. Lancet 2011, 378, 1396–1407.
- Van Poucke, S.; Huskens, D.; Van der Speeten, K.; Roest, M.; Lauwereins, B.; Zheng, M.H.; Dehaene, S.; Penders, J.; Marcus, A.; Lancé, M. Thrombin generation and platelet activation in cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy—A prospective cohort study. PLoS ONE 2018, 13, e0193657.
- Hurdle, H.; Bishop, G.; Walker, A.; Moazeni, A.; Paloucci, E.O.; Temple, W.; Mack, L.; Shing, M. Coagulation after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: A retrospective cohort analysis. Can. J. Anaesth. 2017, 64, 1144–1152.
- Wang, S.; Zhang, Q.; Chen, L.; Liu, G.; Liu, P.F. Thromboelastography-guided blood transfusion during cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Study protocol for a prospective randomised controlled trial. BMJ Open 2020, 10, e042741.
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Sloothen, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743.
- Verwaal, V.J.; Bruin, S.; Boot, H.; van Slooten, G.; van Tinteren, H. 8-Year Follow-up of Randomized Trial: Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy Versus Systemic Chemotherapy in Patients with Peritoneal Carcinomatosis of Colorectal Cancer. Ann. Surg. Oncol. 2008, 15, 2426–2432.
- Nagourney, R.A.; Evans, S.; Tran, P.H.; Nagourney, A.J.; Sugarbaker, P.H. Colorectal cancer cells from patients treated with FOLFOX or CAPOX are resistant to oxaliplatin. Eur. J. Surg. Oncol. 2021, 47, 738–742.
- Auer, R.C.; Sivajohanathan, D.; Biagi, J.; Conner, J.; Kennedy, E.; May, T. Indications for hyperthermic intraperitoneal chemotherapy with cytoreductive surgery: A clinical practice guideline. Curr. Oncol. 2020, 27, 146–154.
- The Chicago Consensus on peritoneal surface malignancies: Management of appendiceal neoplasms. Cancer 2020, 126, 2525–2533.
- Lemoine, L.; Sugarbaker, P.; Van der Speeten, K. Drugs, doses, and durations of intraperitoneal chemotherapy: Standardising HIPEC and EPIC for colorectal, appendiceal, gastric, ovarian peritoneal surface malignancies and peritoneal mesothelioma. Int. J. Hyperth. 2017, 33, 582–592.
- The Chicago Consensus on peritoneal surface malignancies: Management of colorectal metastases. Cancer 2020, 126, 2534–2540.
- Turaga, K.; Levine, E.; Barone, R.; Sticca, R.; Petrelli, N.; Lambert, L.; Nash, G.; Morse, M.; Adbel-Misih, R.; Alexander, H.R.; et al. Consensus guidelines from The American Society of Peritoneal Surface Malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann. Surg. Oncol. 2014, 21, 1501–1505.
- Baumgartner, J.M.; Srivastava, A.; Melnitchouk, N.; Drage, M.G.; Huber, A.R.; Gonzalez, R.S.; Bell, P.; Wu, E.; Resnick, M.; Turaga, K.; et al. A Multi-institutional Study of Peritoneal Recurrence Following Resection of Low-grade Appendiceal Mucinous Neoplasms. Ann. Surg. Oncol. 2021, 28, 4685–4694.
- Jimenez, W.; Sardi, A.; Nieroda, C.; Sittig, M.; Milovanov, V.; Nunez, M.; Aydin, N.; Gushchin, V. Predictive and prognostic survival factors in peritoneal carcinomatosis from appendiceal cancer after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2014, 21, 4218–4225.
- Sugarbaker, P.H. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: Progress toward a new standard of care. Cancer Treat. Rev. 2016, 48, 42–49.
- The Chicago Consensus on peritoneal surface malignancies: Palliative care considerations. Cancer 2020, 126, 2571–2576.
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; Bremers, A.J.A.; Burger, J.W.A.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770.
- Goéré, D.; Glehen, O.; Quenet, F.; Guilloit, J.M.; Bereder, J.M.; Lorimier, G.; Thibaudeau, E.; Ghouti, L.; Pinto, A.; Tuech, J.J.; et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): A randomised, phase 3 study. Lancet Oncol. 2020, 21, 1147–1154.
- Sugarbaker, P.H.; Graves, T.; DeBruijn, E.A.; Cunliffe, W.J.; Mullins, R.E.; Hull, W.E.; Oliff, L.; Schlag, P. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: Pharmacological studies. Cancer Res. 1990, 50, 5790–5794.
- Cashin, P.H.; Mahteme, H.; Spång, N.; Syk, I.; Frödin, J.E.; Torkzad, M.; Glimelius, B.; Graf, W. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: A randomised trial. Eur. J. Cancer 2016, 53, 155–162.
- Sugarbaker, P.H. Normothermic intraperitoneal chemotherapy long term (NIPEC-LT) in the management of peritoneal surface malignancy, an overview. Pleura Peritoneum 2017, 2, 85–93.
- Sugarbaker, P.H.; Chang, D. Long-term regional chemotherapy for patients with epithelial malignant peritoneal mesothelioma results in improved survival. Eur. J. Surg. Oncol. 2017, 43, 1228–1235.
- Sugarbaker, P.H. Intraperitoneal delivery of chemotherapeutic agents for the treatment of peritoneal metastases: Current challenges and how to overcome them. Expert Opin. Drug Deliv. 2019, 16, 1393–1401.
- Yonemura, Y.; de Aretxabala, X.; Fujimura, T.; Fushida, S.; Katayama, K.; Bandou, E.; Sugiyama, K.; Kawamura, T.; Kinoshita, K.; Endou, Y.; et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: Final results of a randomized controlled study. Hepatogastroenterology 2001, 48, 1776–1782.
- Huang, J.Y.; Xu, Y.Y.; Sun, Z.; Zhu, Z.; Song, Y.X.; Guo, P.T.; You, Y.; Xu, H.M. Comparison different methods of intraoperative and intraperitoneal chemotherapy for patients with gastric cancer: A meta-analysis. Asian Pac. J. Cancer Prev. 2012, 13, 4379–4385.
- Farrell, R.; Burling, M.; Lee, Y.C.; Pather, S.; Robledo, K.; Mercieca-Bebber, R.; Stockler, M. Clinical Trial Protocol for HyNOVA: Hyperthermic and Normothermic intraperitoneal chemotherapy following interval cytoreductive surgery for stage III epithelial OVArian, fallopian tube and primary peritoneal cancer (ANZGOG1901/2020). J. Gynecol. Oncol. 2021.
- Alyami, M.; Hübner, M.; Grass, F.; Bakrin, N.; Villeneuve, L.; Laplace, N.; Passot, G.; Glehen, O.; Kepenekian, V. Pressurised intraperitoneal aerosol chemotherapy: Rationale, evidence, and potential indications. Lancet Oncol. 2019, 20, e368–e377.
- Kusamura, S.; Azmi, N.; Fumagalli, L.; Baratti, D.; Guaglio, M.; Cavalleri, A.; Garrone, G.; Battaglia, L.; Barretta, F.; Deraco, M. Phase II randomized study on tissue distribution and pharmacokinetics of cisplatin according to different levels of intra-abdominal pressure (IAP) during HIPEC (NCT02949791). Eur. J. Surg. Oncol. 2021, 47, 82–88.
- Nadiradze, G.; Horvath, P.; Sautkin, Y.; Archid, R.; Weinreich, F.J.; Königsrainer, A.; Reymond, M.A. Overcoming Drug Resistance by Taking Advantage of Physical Principles: Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). Cancers 2019, 12, 34.
- Giger-Pabst, U.; Bucur, P.; Roger, S.; Falkenstein, T.A.; Tabchouri, N.; Le Pape, A.; Lerondel, S.; Demtröder, C.; Salamé, E.; Ouaissi, M. Comparison of Tissue and Blood Concentrations of Oxaliplatin Administrated by Different Modalities of Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2019, 26, 4445–4451.
- Göhler, D.; Große, S.; Bellendorf, A.; Falkenstein, T.A.; Ouaissi, M.; Zieren, J.; Stintz, M.; Giger-Pabst, U. Hyperthermic intracavitary nanoaerosol therapy (HINAT) as an improved approach for pressurised intraperitoneal aerosol chemotherapy (PIPAC): Technical description, experimental validation and first proof of concept. Beilstein J. Nanotechnol. 2017, 8, 2729–2740.
- Christou, N.; Auger, C.; Battu, S.; Lalloué, F.; Jauberteau-Marchan, M.O.; Hervieu, C.; Verdier, M.; Mathonnet, M. Intraperitoneal Chemotherapy for Peritoneal Metastases: Technical Innovations, Preclinical and Clinical Advances and Future Perspectives. Biology 2021, 10, 225.
- Gong, Y.; Wang, P.; Zhu, Z.; Zhang, J.; Huang, J.; Wang, T.; Chen, J.; Xu, H. Benefits of Surgery After Neoadjuvant Intraperitoneal and Systemic Chemotherapy for Gastric Cancer Patients with Peritoneal Metastasis: A Meta-Analysis. J. Surg. Res. 2020, 245, 234–243.
- Prabhu, A.; Brandl, A.; Wakama, S.; Sako, S.; Ishibashi, H.; Mizumoto, A.; Takao, N.; Noguchi, K.; Motoi, S.; Ichinose, M.; et al. Neoadjuvant Intraperitoneal Chemotherapy in Patients with Pseudomyxoma Peritonei-A Novel Treatment Approach. Cancers 2020, 12, 2212.
- Yonemura, Y.; Bandou, E.; Sawa, T.; Yoshimitsu, Y.; Endou, Y.; Sasaki, T.; Sugarbaker, P.H. Neoadjuvant treatment of gastric cancer with peritoneal dissemination. Eur. J. Surg. Oncol. 2006, 32, 661–665.
- Yonemura, Y.; Elnemr, A.; Endou, Y.; Ishibashi, H.; Mizumoto, A.; Miura, M.; Li, Y. Effects of neoadjuvant intraperitoneal/systemic chemotherapy (bidirectional chemotherapy) for the treatment of patients with peritoneal metastasis from gastric cancer. Int. J. Surg. Oncol. 2012, 2012, 148420.
- Ishigami, H.; Fujiwara, Y.; Fukushima, R.; Nashimoto, A.; Yabusaki, H.; Imano, M.; Imamoto, H.; Kodera, Y.; Uenosono, Y.; Amagai, K.; et al. Phase III Trial Comparing Intraperitoneal and Intravenous Paclitaxel Plus S-1 Versus Cisplatin Plus S-1 in Patients With Gastric Cancer With Peritoneal Metastasis: PHOENIX-GC Trial. J. Clin. Oncol. 2018, 36, 1922–1929.
- Zhang, X.; Huang, H.; Wei, Z.; Zhu, Z.; Yang, D.; Fu, H.; Xu, J.; Hu, Z.; Zhang, Y.; You, Q.; et al. Comparison of Docetaxel + Oxaliplatin + S-1 vs Oxalipatin + S-1 as Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: A Propensity Score Matched Analysis. Cancer Manag. Res. 2020, 12, 6641–6653.
- Waite, K.; Youssef, H. The Role of Neoadjuvant and Adjuvant Systemic Chemotherapy with Cytoreductive Surgery and Heated Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Systematic Review. Ann. Surg. Oncol. 2017, 24, 705–720.
- Beeharry, M.K.; Ni, Z.T.; Yang, Z.Y.; Zheng, Y.N.; Feng, R.H.; Liu, W.T.; Yan, C.; Yao, X.X.; Li, C.; Yan, M.; et al. Study protocol of a multicenter phase III randomized controlled trial investigating the efficiency of the combination of neoadjuvant chemotherapy (NAC) and neoadjuvant laparoscopic intraperitoneal hyperthermic chemotherapy (NLHIPEC) followed by R0 gastrectomy with intraoperative HIPEC for advanced gastric cancer (AGC): Dragon II trial. BMC Cancer 2020, 20, 224.
- Fujimoto, S.; Shrestha, R.D.; Kokubun, M.; Kobayashi, K.; Kiuchi, S.; Takahashi, M.; Konno, C.; Ohta, M.; Koike, S.; Kitsukawa, Y.; et al. Clinical trial with surgery and intraperitoneal hyperthermic perfusion for peritoneal recurrence of gastrointestinal cancer. Cancer 1989, 64, 154–160.
- Elias, D.; Bonnay, M.; Puizillou, J.M.; Antoun, S.; Demirdjian, S.; El, O.A.; Pignon, J.P.; Drouard-Troalen, L.; Ouellet, J.F.; Ducreux, M. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: Pharmacokinetics and tissue distribution. Ann. Oncol. 2002, 13, 267–272.
- Van der Speeten, K.; Stuart, O.A.; Mahteme, H.; Sugarbaker, P.H. Pharmacology of perioperative 5-fluorouracil. J. Surg. Oncol. 2010, 102, 730–735.
- Yurttas, C.; Hoffmann, G.; Tolios, A.; Haen, S.P.; Schwab, M.; Konigsrainer, I.; Konigsrainer, A.; Beckert, S.; Loffler, M.W. Systematic Review of Variations in Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Metastasis from Colorectal Cancer. J. Clin. Med. 2018, 7, 567.
- Levine, E.A.; Votanopoulos, K.I.; Shen, P.; Russell, G.; Fenstermaker, J.; Mansfield, P.; Bartlett, D.; Stewart, J.H. A Multicenter Randomized Trial to Evaluate Hematologic Toxicities after Hyperthermic Intraperitoneal Chemotherapy with Oxaliplatin or Mitomycin in Patients with Appendiceal Tumors. J. Am. Coll. Surg. 2018, 226, 434–443.
- Glockzin, G.; von Breitenbuch, P.; Schlitt, H.J.; Piso, P. Treatment-related morbidity and toxicity of CRS and oxaliplatin-based HIPEC compared to a mitomycin and doxorubicin-based HIPEC protocol in patients with peritoneal carcinomatosis: A matched-pair analysis. J. Surg. Oncol. 2013, 107, 574–578.
- Glockzin, G.; Gerken, M.; Lang, S.A.; Klinkhammer-Schalke, M.; Piso, P.; Schlitt, H.J. Oxaliplatin-based versus irinotecan-based hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal metastasis from appendiceal and colorectal cancer: A retrospective analysis. BMC Cancer 2014, 14, 807.
- Quenet, F.; Goéré, D.; Mehta, S.S.; Roca, L.; Dumont, F.; Hessissen, M.; Saint-Aubert, B.; Elias, D. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann. Surg. 2011, 254, 294–301.
- Sipok, A.; Sardi, A.; Nieroda, C.; King, M.C.; Sittig, M.; Gushchin, V. Comparison of Survival in Patients with Isolated Peritoneal Carcinomatosis from Colorectal Cancer Treated with Cytoreduction and Melphalan or Mitomycin-C as Hyperthermic Intraperitoneal Chemotherapy Agent. Int. J. Surg. Oncol. 2018, 2018, 1920276.
- Frøysnes, I.S.; Andersson, Y.; Larsen, S.G.; Davidson, B.; Øien, J.T.; Julsrud, L.; Fodstad, Ø.; Dueland, S.; Flatmark, K. ImmunoPeCa trial: Long-term outcome following intraperitoneal MOC31PE immunotoxin treatment in colorectal peritoneal metastasis. Eur. J. Surg. Oncol. 2021, 47, 134–138.
- Frøysnes, I.S.; Andersson, Y.; Larsen, S.G.; Davidson, B.; Øien, J.T.; Olsen, K.H.; Giercksky, K.E.; Julsrud, L.; Fodstad, Ø.; Dueland, S.; et al. Novel Treatment with Intraperitoneal MOC31PE Immunotoxin in Colorectal Peritoneal Metastasis: Results From the ImmunoPeCa Phase 1 Trial. Ann. Surg. Oncol. 2017, 24, 1916–1922.
- Feng, J.; Li, K.; Liu, G.; Feng, Y.; Shi, H.; Zhang, X. Precision hyperthermia-induced miRNA-409-3p upregulation inhibits migration, invasion, and EMT of gastric cancer cells by targeting KLF17. Biochem. Biophys. Res. Commun. 2021, 549, 113–119.
- Sugarbaker, P.H.; Stuart, O.A. Pharmacokinetics of the intraperitoneal nanoparticle pegylated liposomal doxorubicin in patients with peritoneal metastases. Eur. J. Surg. Oncol. 2021, 47, 108–114.
- Wu, H.T.; Peng, K.W.; Ji, Z.H.; Sun, J.H.; Zhang, Q.; Yang, X.J.; Huang, C.Q.; Li, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with lobaplatin and docetaxel to treat synchronous peritoneal carcinomatosis from gastric cancer: Results from a Chinese center. Eur. J. Surg. Oncol. 2016, 42, 1024–1034.
- Wu, H.T.; Yang, X.J.; Huang, C.Q.; Sun, J.H.; Ji, Z.H.; Peng, K.W.; Zhang, Q.; Li, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with lobaplatin and docetaxel improves survival for patients with peritoneal carcinomatosis from abdominal and pelvic malignancies. World J. Surg. Oncol. 2016, 14, 246.
- Larsen, A.K.; Trindade, C.; Bouygues, A.; Louadj, L.K.; Thouroude, S.K.; Klinz, S.G.; Kalra, A.; Henriques, J.; Chibaudel, B.; Gramont, A.D.; et al. Influence of liposomal irinotecan (nal-IRI) and non-liposomal irinotecan, alone and in combination, on tumor growth and angiogenesis in colorectal cancer (CRC) models. J. Clin. Oncol. 2018, 36, 711.
- Morton, D.L.; Ollila, D.W.; Hsueh, E.C.; Essner, R.; Gupta, R.K. Cytoreductive surgery and adjuvant immunotherapy: A new management paradigm for metastatic melanoma. CA Cancer J. Clin. 1999, 49, 101–116.
- Thadi, A.; Khalili, M.; Morano, W.F.; Richard, S.D.; Katz, S.C.; Bowne, W.B. Early Investigations and Recent Advances in Intraperitoneal Immunotherapy for Peritoneal Metastasis. Vaccines 2018, 6, 54.
- Morano, W.F.; Aggarwal, A.; Love, P.; Richard, S.D.; Esquivel, J.; Bowne, W.B. Intraperitoneal immunotherapy: Historical perspectives and modern therapy. Cancer Gene Ther. 2016, 23, 373–381.
- Ströhlein, M.A.; Heiss, M.M.; Jauch, K.W. The current status of immunotherapy in peritoneal carcinomatosis. Expert Rev. Anticancer Ther. 2016, 16, 1019–1027.
