2. The Role of OGG1 in DNA Oxidative Modification
Oxidative stress can exacerbate airway inflammatory responses by which asthma, chronic obstructive pulmonary disease (COPD) and other invasive pulmonary diseases are accompanied [
22,
23]. Free radicals and reactive oxygen species can attenuate the mucosal function of organs, increase endothelial permeability, reduce endothelial adhesion and affect the reconstruction of extracellular matrix. In addition to the direct action of oxidants, the oxidative stress response can also aggravate the inflammatory response through the following mechanisms: (1). Oxidants can weaken the deformation ability of neutrophils, which causes the retention and activation of neutrophils in pulmonary microcirculation [
24]; (2). Oxidative stress activates transcription factor nuclear factor κB (NF-κB) and activator protein 1 (AP-1), which regulate the release of inflammatory mediators, aggravating inflammation [
25]; (3). Oxidants can promote the expression of adhesion molecules on the surface of neutrophils. Neutrophils recruited in the lungs release active oxygen and protein amines after activation, causing tissue damage at the site of inflammation [
26]. Currently, most studies focus on changes in protein function or abnormal lipid metabolism under oxidative stress, whereas the mechanism of DNA oxidative modification on inflammatory response is not clear.
In the course of evolution, organisms evolved complex repair pathways that maintain genome integrity and accuracy, such as base excision repair (BER), nucleotide excision repair (NER), mismatch repair (MMR), homologous recombination (HR) and non-homologous termination (NHEJ) [
27,
28,
29,
30,
31]. When facing stimulation, DNA oxidative damage could still accumulate despite the existence of multiple repair pathways, causing gene mutations or cell death [
32,
33].
DNA is oxidized to produce oxidative damage leading to modified bases (oxidized bases), chain break (DNA single and double chain break) and chain cross [
34]. DNA oxidative modification has site specificity. Among all four bases—cytosine, guanine, adenine and thymine, the lowest redox potential makes guanine (G) the most fragile base for oxidative stress and 7, 8-dihydro-8-oxoguanine (8-oxoG) the most common DNA oxidative damage; thus, the appearance and accumulation of 8-oxoG in DNA sequence was also considered as the biomarker of DNA oxidative damage. As is mentioned above, 8-oxoG accumulation is usually closely related to many physiological processes, and due to the stacking of π-π bonds and the interaction of electron orbitals, the ionization energy of 5’ terminal guanine in continuous guanine is reduced, so that the 5’ ends containing multiple adjacent guanines, for example, 5’G < 5’GG < 5’GGG, could be the site of 8-oxoG preferentially [
35]. The DNA double helix is not affected by 8-oxoG, but it can mismatch with adenine (A) and appear GC→TA mutation in DNA replication [
36].
In eukaryotes, this DNA oxidative modification can be identified by the DNA glycosylation enzyme, which can repair 8-oxoG by means of base excision repair (BER) [
37]. As the initiator of BER process, OGG1 plays a pivotal role in the removal of 8-oxoG and formamidopyrimidine (Fapy-G), the ring-opened guanine [
37].
The base excision repair process could be divided into two sections, the AP sites formation and the resolution of AP sites, executed by multiple enzymes. DNA glycosylation enzymes cut off the
N-glycosyl bonds by hydrolase activity and remove specific damage bases to produce base-free sites (AP sites), which are further treated with endonuclease and ligase. Finally, the complete repair of the original DNA sequence is realized. There are five DNA ribozymes specifically recognizing oxidized bases in mammalian cells. They are divided into two families: the Nth family including OGG1 and NTH1, and Nei family including NEIL1, NEIL2 and NEIL3 [
38]. These two families are named after homologous proteins in bacteria—endonuclease III (Nth) and endonuclease VIII (Nei) [
39,
40,
41], and each have different mechanisms of de-base lyase reaction. OGG1 and NTH1 cut the DNA chain by β-lyase activity, producing 3’ dRP and 5’ P ends, whereas NEIL1/2 have βδ lyase activity, thus creating 3’ P and 5’ P in the chain gap [
42].
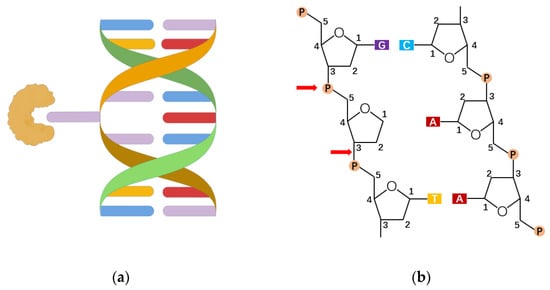
The mechanism of NEIL3 catalysis needs further study. So far, 8-hydroxyguanine DNA glucosidase 1 (OGG1) is a DNA repair enzyme found mainly in mammals that identifies and cleaves 8-oxoG on genomic DNA. OGG1 corrects the occurrence of 8-oxoG through a series of complex and subtle repair pathways to maintain genome accuracy. In the process, OGG1 binds to DNA containing damaged bases and bends the DNA strands, from which 8-oxoG is exposed and encapsulated in an OGG1 highly conserved catalytic active pocket, which thus cuts off the
N-glycoside bond [
43,
44,
45]. Among them, the binding ability of the OGG1 active pocket to 8-oxoG is about 105 times higher than that of peripheral pocket to guanine, which means the damaged base first enters the secondary center after OGG1 has been extruded into a double helix and then inserts itself into the active pocket (
Figure 2a) [
37,
46,
47,
48]. This explains why OGG1 can accurately distinguish between highly similar 8-oxoG and G. The mechanism of OGG1 repair of 8-oxoG is summarized as follows: OGG1 removes the
N-glycoside bond of the damaged base through the above recognition process, acting on a sugar-phosphate backbone to form an apurinic/apyrimidinic (AP)-site(s) (
Figure 2b). Then, using its own weak AP lyase, OGG1 shows β-elimination thereby forming 5’ P (phosphate) and 3’ d RP (unsaturated hydroxyl aldehyde) ends. Because the 3’ end residue does not make a normal connection, OGG1 recruits apurinic/apyrimidinic endonuclease 1 (APE1) to clean up the 3’ end and get 3’-OH ends. At this point, DNA polymerase β inserts the correct nucleotide through the polymerization and DNA ligase III, and DNA ligase I connects the DNA chains, completing the base excision repair initiated by the OGG1 [
42].
Figure 2. The recognition and excision of 8-oxoG. (a) The lesion-specific binding pocket of OGG1 allows the recognition of the extrahelical flipped 8-oxoG, and excises it from DNA strand (drawn by Figdraw). (b) OGG1 acts on a sugar-phosphate backbone to form AP-sites, generating 5’ P ends and 3’ d RP ends.
3. The Roles of Base Excision Repair Enzyme OGG1 in Gene Expression
Guanine is readily oxidized, thereby disrupting the integrity of the genome. From an evolutionary perspective, vertebrate genomes contain more guanine than other organisms. OxiDIP-Seq of human MCF10A cells shows that 8-oxoG is significantly enriched in the intergenic region and the intron region of gene body, and most of the 8-oxoG are enriched in protein coding DNA [
49]. In total, 72% of human promoter regions are spread with higher GC base pairs [
50], including gene-coding pro-inflammatory factors, proto-oncogenes and growth factors. Some conserved sequences recognized by transcription factors are also enriched with guanine. For example, transcription factor specificity protein 1 (SP-1) recognizes GC-rich sequences 5’-GGGGCGGGG -3’, and κB sequences identified by NF-κB have a 5’-GGGRNYYYCC-3’ pattern, of which 5’ ends are distributed with continuous guanine [
51]. In recent years, there is growing evidence that 8-oxoG and its specific repair protein OGG1 have epigenetic regulatory effects on gene transcription. Some researchers found that Ogg1-/-mice shows significant 8-oxoG accumulation; however, it does not lead to cancer or affect life span and embryo development. Surprisingly, the inflammatory response in Ogg1-/-mice was also slighter [
52], indicating that OGG1 function is closely related to promoting inflammatory cell recruitment and promoting cytokine expression.
Experimental evidence was widely reported in recent years. Researchers found an abnormal increase in 8-oxoG accumulation and a high spontaneous rate of lung cancer in the genome of Ogg1-/-mice [
52]. The reduced immune response of Ogg1-/-mice models to endotoxic shock, diabetes and hypersensitivity manifested as decreased neutrophil infiltration and lower expressions of Th1 and Th2 cytokines [
53]. Moreover, inflammation of the Ogg1-/-mice stomach was slighter after H. pylori infection when comparing to wildtype. Similarly, in an asthma model, the cell infiltration in the airway, perivascular and alveoli and the secretion of Th1, Th2 and Th17 cytokines of Ogg1-/-mice were slighter [
54].
The small molecule inhibitor of OGG1,TH5487 is an effective selective active site binding agent, which inhibits the activity of OGG1 DNA glycosylase, so OGG1 cannot insert itself into the DNA chain and join the 8-oxoG, and also reduces the DNA occupation of NF-κB and the level of TNFα-induced neutrophil recruitment and various cytokines in human bronchial epithelial cells and in mice models [
55]. In 2019, Pan et al. [
56] showed that in lung inflammation challenged by ozone, levels of 8-oxoG in genomes gradually ascend. HE staining showed that with the prolongation of ozone stress, more inflammatory cells infiltrated airways around the alveoli, and other manifestations such as airway stenosis, partial alveolar rupture and enlarged alveolar cavity arose. After five days of ozone stress, 8-oxoG accumulated in the airway epithelium and showed an increased oxidation level of protein. The OGG1 inhibitor TH5487 treatment significantly improved above phenomenon and manifested as reduced infiltration of inflammatory cells in the airways, surrounding alveoli and the complete alveolar structure. There are also other studies reporting small molecular inhibitors designed for OGG1, which provide a bright outlook for clinical anti-inflammatory treatment [
57].
The aforementioned studies suggest that OGG1 has immunomodulatory functions, which are closely related to promoting inflammatory cell recruitment and cytokine expression. Studying the role of 8-oxoG and its repair enzyme OGG1 in pulmonary inflammation plays an important role in elucidating the mechanism of pulmonary inflammation and developing new therapeutic targets for chronic pulmonary inflammation from the perspective of oxidative stress.
4. Roles of OGG1 in Pulmonary Inflammation and Disease
4.1. The Roles of OGG1 in Lung Cancer
Lung cancer ranks as one of the cancers with the highest morbidity and mortality in recent years, which is also widely reported to have close association with oxidative stress. Interestingly, there have been studies over the role of OGG1 in lung cancer separated into different directions at the very beginning.
Case-control studies carried out in populations of different diseases, regions or life states, together with meta-analysis based on them, tried to figure out the correlation between OGG1 and lung cancers, and the conclusions were inconsistent. Some researchers summarized that OGG1 has no relation to a higher risk of lung cancer [
97,
98], or if the association appeared in certain populations, such as in non-smokers [
99]. Opposite opinions suggest that the Cys/Cys genotype of the OGG1 Ser326Cys polymorphism was believed to have an association with a lung cancer risk [
100], and the combined OGG1-Cys/Cys and Ser/Cys genotypes show a 1.93-fold increased risk of lung cancer, which was particularly elevated among women who suffered from relatively high cumulative exposure to smoky coal [
101]. Besides, genome-wide association studies (GWASs) found that the genotypes for two DNA repair genes, TP53 and OGG1, showed significant associations with lung squamous cell carcinoma (SQC) risk [
102].
4.2. The Roles of OGG1 in Innate Lung Immunity
Airway epithelium is the main surface in contact with inhaled particles, pathogens and allergens, and is lined with a semi-impermeable barrier of highly adapted epithelial cells [
109]. Epithelial cells play a central role in triggering the protective host response. After microbial invasion, airway epithelial cells first activate the reproductive system coding Pattern recognition receptor, recognize the pathogen-associated molecular pattern (PAMPs) or damage-associated pattern (DamPs), and then trigger cells to produce innate immunity (IIR) to prevent/reduce the spread of foreign pathogens, which is then key to triggering adaptive immunity [
110].
Ros produced in the PRR signaling pathway plays an important role in signal transduction by controlling phosphorylation. Little is known about the synergistic effects in IIR of cellular reactive oxygen species (ROS). It was proposed that OGG1 and Ataxia telangiectasia-mutated (ATM) are endogenous nuclear ROS sensors that coordinate the Pattern recognition receptor signaling pathway and regulate the innate immune response [
95]. ATM responded to DSBs/ROS, forming a scaffold with ribosomal S6 kinase, which induced RelA phosphorylation and resulted in the transcription coupling of type I and III IFN, CC and CXC chemokines. The cytosolic OGG1-8-oxoG complex acts as a guanine nucleotide exchange factor, inducing the formation of MAP-, PI3-and MS-kinases, and activating the classical NF-kB pathway by phosphorylation and nuclear translocation at Ser276, and playing a role in innate immune response (IIR).
4.3. The Roles of OGG1 in Airway Remodeling and Asthma
Genes under regulation of the OGG1-8-oxoG complex are associated with some important biological processes and airway remodeling. Researchers performed an imitation of the OGG1-BER process by attacking mice lungs using 8-oxoG. In total, 1592 transcripts were identified by RNA-seq analysis [
103]. The up-regulated mRNA was associated with biological processes, including homeostasis, immune system, macrophage activation, surface tension regulation and response to stimuli. These processes are mediated by chemokine, cytokine, gonadotropin-releasing hormone receptor, integrin and interleukin signaling pathways. In addition, their analysis of another study identified 3252 differentially expressed transcripts, of which 2435 were up-regulated and 817 were down-regulated [
102]. In the up regulated transcripts, 2080 mRNA were identified, encoding proteins involved in the regulation of actin family cytoskeleton, extracellular matrix, cell adhesion, cadherin and cell junctions that influence biological processes such as tissue development, cell-to-cell adhesion, cell communication and the immune system. OGG1-BER involved in the overexpression of cadherin, integrin, Rho GTP enzyme, TGF, WNT and cytokine/chemokine signaling pathway indicates that OGG1-BER can continuously repair DNA oxidative damage and conduct downstream signal transduction through small GTP enzyme and induce airway remodeling associated with gene expression, leading to changes in lung function and structure.
Asthma is characterized by airway inflammation and hyperresponsiveness, and the tilt towards TH2 cytokines is considered to be a key risk factor for asthma. There is growing evidence that oxidative stress is also a key factor in the development of asthma. Oxidative stress levels in the lungs of asthmatics are increased and ROS produced in the cells regulates the gene expression of asthma-associated TH2 cytokine IL-4 [
111]. In addition, oxidative DNA damage in the peripheral blood lymphocyte was significantly higher in asthmatic patients than in healthy subjects [
112]. Whole-genome expression analysis also suggests that the OGG1-BER-induced gene expression may play a role in asthma and EIA Pathophysiology [
113,
114]. OGG1 was found to up-regulate the expression of Cytokines, especially IL-4, through the STAT6/NF-κB pathway in OVA-sensitized asthmatic mice [
54].
4.4. The Roles of OGG1 in Allergic Airway Inflammation
Ragweed pollen extract (PWPE), which contains NADPH Oxidase (Nox; RWPENOX), can induce the production of a reactive oxygen species (ROS) and lead to airway hypersensitivity. Bacsi et al. found that the level of 8-oxoG increased in the airway epithelial cells of mice challenged with RWPE, and DNA SSB formed during 8-oxoG repairment enhanced antigen-driven allergic immune response [
115]. It was found that attacking OGG1 proficient mice with ragweed pollen induced strong recruitment of airway eosinophil granulocyte, whereas attacking OGG1 deficient mice showed reduced recruitment [
116]. After infusing free 8-oxoG into mouse lungs to simulate the OGG1-BER pathway and evaluate the unbiased RNA sequencing and molecular histologic changes, researchers found that PWPE attack could induce an oxidative burst, cause DNA damage and activate the OGG1 signal, resulting in the differential expression of 84 microRNAs (miRNAs). OGG1 can increase levels of TH2 cytokines (such as IL-13, IL-4 and IL-5) by downregulating miRNA let-7b-3p, which leads to Eosinophil granulocyte recruitment and exacerbates allergic airway inflammation. Affymetrix microarray genechips show that in airway epithelial cells, the OGG1-Ras regulatory network is regulated by TF, including NF-κB, tumor protein-53 (TP53), Kruppel-like factor 4 (KLF4) and so on [
117]. It is suggested that the recruitment of OGG1-BER-small GTPases, activated TF and/or OGG1-mediated recruitment of TF in promoter regions may be involved in miRNA transcription regulation. The aforesaid research suggest that it is worth exploiting a new therapy to alleviate airway inflammation achieved by pharmacological modulation of OGG1 signal transduction or local administration of specific miRNAs.
4.5. The Roles of OGG1 in Hyperoxia-Induced Lung Injury
In clinical work, oxygen was given as a supportive treatment for patients suffering from acute respiratory distress syndrome (ARDS). However, hyperoxia could also induce lung injury. Considering its function as a key factor in the response to oxidative DNA damage, it is natural to consider of the role of OGG1. The existence of OGG1 could help elevate the resistance to hyperoxic cytotoxicity, overexpression of hOGG1, alleviate DNA damage of A549 and AECII from hyperoxia and H
2O
2 exposure, and could be related to the MAPK pathway activation [
118,
119]. It has been reported that inflammatory cytokines (TNF-α,IL-6,IFN-γ) increased in the OGG1 deficiency in mice after hyperoxia exposure [
120], as OGG1 interacted with the promoter of Atg7, thus regulating the autophagy pathway to influence inflammatory cytokine release in the hyperoxia-induced lung injury.
Oxidative stress has been testified to be a non-negligible risk factor for neonatal disease, especially affecting pulmonary development. Supplementary oxygen for respiratory disfunction in infants is an important source of oxidative stress, causing lung injury that manifests as delayed alveolar growth, extracellular matrix deposition and pulmonary fibrosis, which could evolve into permanent lung injury—bronchopulmonary dysplasia (BPD). Human lung morphogenesis is divided into five stages: embryonic, pseudoglandular, canalicular, saccular and alveolar. Premature infants are under a higher risk of being attacked by supplementary oxygen, since their lungs tend to be at the saccular-alveolar stage, during which lungs are more sensitive to oxidative stress [
121]. Elevated 8-OHdG levels along with lung injury were observed in a hyperoxia model, and its alleviation after suppressing oxidative stress implied the potential role of OGG1 in pulmonary development [
122], which was subsequently documented by another research team, who suggested that OGG1 expression was upregulated in a hyperoxia-induced BPD model [
123]. Interestingly, the expression of OGG1 showed a time-related fluctuation, which reached a peak at a certain timepoint but descended to normal when hyperoxia exposure was prolonged.