A biofilm is a community of microorganisms, especially bacteria, that maintains an organized and structured strategy for growing and proliferating on any surface for their survival. The organization of the cells among the bacteria in the biofilm has primarily been revealed by examining single species. The survival of the bacteria present in the biofilm is feasible because of their orientation in the form of microcolonies, which are encapsulated in the extracellular polymeric substance of the matrix. These are separated by open water channels that serve as the primordial circulatory system for the transportation of nutrients and the disposal of metabolic waste products. All the specific bacteria maintain a microenvironment controlling the pH, nutrient availability, and temperature, which influence the biofilm growth. Biofilm maturation is a multi-stage developmental process with distinct characteristics that should be taken into account when developing antibiotic treatment regimens for biofilms. Biofilms have been extensively accepted as one of the main causes responsible for human diseases.
1. Pathogen of Biofilm
1.1. Bacterial Type and Importance of One Health Approach
In the formation of biofilm, the involvement of microorganisms is diverse and takes many forms. Various types of microbes play a vital role in the formation of biofilm. As a consequence of this, the type of pathogen is a prime factor that controls the biofilm formation effectively. Together, both Gram-positive and Gram-negative microorganisms participate in biofilm formation [
10]. Bacteria usually show the ability to bind with substrates due to high adherence potential, and they form biofilms that manifest two kinds of living states: one is a free-living, planktonic living state and the other one is a sessile living state [
11]. Some bacterial types have the advantage of forming a biofilm, as this phenomenon is considered to be one of the methods for microbial self-defence. This formation, in due course, helps the cells of the biofilm to remain in a favourable condition that creates a niche in which they exist. Bacterial cells are thought to grow naturally in close proximity to one another [
12]. An innumerable number of bacteria, including Gram-positive and Gram-negative bacteria, control the formation of biofilm. Gram-positive bacteria include
Bacillus spp.,
Listeria monocytogenes,
Staphylococcus spp., and lactic acid bacteria, such as
Lactobacillus plantarum and
Lactococcus lactis. Gram-negative bacteria include
Escherichia coli,
Klebsiella pneumoniae,
Pseudomonas aeruginosa, and
Acinetobacter baumannii, which are responsible for various pathological diseases [
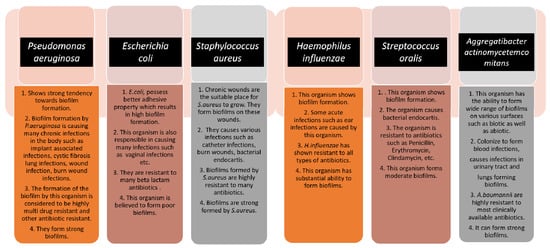
13]. Biofilm formation is very prominent in implant-associated infections as well. The majority of the implant-associated infections emerge from biofilm formation, which is difficult to treat with the conventional methods of antibiotics.
Staphylococcus aureus and
Streptococcus oralis are Gram-positive bacteria found to cause implant-associated infections.
Pseudomonas aeruginosa and
Aggregatibacter actinomycetemcomitans are Gram-negative bacteria that play a vital role in generating implant-associated infections [
14]. The below figure (
Figure 1) gives some information about the different microorganisms, along with the biofilm-associated diseases that they are capable of forming [
15]. Antimicrobial resistance genes circulate across the microbiomes of humans, animals, and the environment, which make up the One Health concept’s many sectors. One Health is defined as a multidisciplinary effort—working locally, nationally, and globally—to achieve optimal health for people, animals, and the environment through policy, research, education, and practice (3). The One Health concept has previously focused on the interconnections and interdependencies among sectors at local sites; however, in light of global health, the understanding of communication among local ecosystems and the identification of factors that contribute to the global AMR crisis have recently received a lot of attention (3). The human microbiomes of the gut, skin, and respiratory tract have their resistomes (a collection of all antimicrobial resistance genes) which are assessed using metagenomics based on next-generation sequencing technologies. Controlling antimicrobial resistance gene flow from other sectors to the human sector, particularly antimicrobial resistance gene transfer to disease-causing bacteria, requires an understanding of the dynamics of the human resistome (the collection of all antimicrobial resistance genes) and its relationships with the other One Health sectors [
16,
17]. Removing contaminated foreign bodies, the selection of biofilm-active, sensitive, and well-penetrating antibiotics, topical antibiotic delivery in high doses and combinations, and anti-quorum sensing or biofilm dispersion agents are all elements of a multidisciplinary partnerships that are taken care of by the One Health Approach [
16].
Figure 1. The schematic diagram highlights the characteristics of each microorganism in terms of biofilm formation and the diseases caused by them. The strength of the biofilm formation and antibiotic resistance are also mentioned.
1.2. Genetic Regulation of Biofilm Formation
In Gram-negative organisms, the genetic regulators of biofilm formation include quorum sensing (QS), bis-(3′-5′)-cyclic diguanosine monophosphate (c-di-GMP), and small RNAs (sRNAs). Quorum sensing (QS) is regarded as an important regulator for intercellular communication. QS is entirely based on small, self-contained, signal-generating molecules known as autoinducers. When there are enough bacteria existing and the accumulation of autoinducers attains a critical mass, the bacteria sense this and respond by suppressing or activating target genes. QS systems are important in the formation and dispersal of bacterial biofilms. Even though these systems are not engaged in the attachment and early stages of biofilm growth, they are required for subsequent biofilm development and are the primary regulators of biofilm dispersal. During the formation and spread of bacterial biofilms, QS systems are extremely crucial. The c-di-GMP signalling network, the second key biofilm regulator, is the most complex secondary signalling system identified in bacteria. Selection among planktonic and biofilm-associated lifestyles is influenced by c-di-GMP [
18]. The synthesis of exopolysaccharides, sticky pili, and adhesins, the secretion of extracellular DNA (e-DNA), and the control of cell death and motility are all factors regulated by c-di-GMP and are critical for three-dimensional biofilm structure development. c-di-GMP regulates bacterial transcription, enzyme activity, and even the function of bigger cellular structures after attaching to a number of cellular receptors. Bacterial biofilm generation and dissemination are tightly regulated processes that are influenced by genetic and environmental factors [
19].
Finally, tiny non-coding RNA molecules, or sRNAs, have been found to play a role in post-transcriptional gene regulation in bacteria, involving metabolic activities, stress adaption, and microbial disease [
20].
2. Biofilm Development
2.1. Initial Adhesion
The initiation of biofilm formation begins with the adhesion of the bacterial cells to any interface or medical device surfaces. Various signalling pathways help the bacteria to recognize the surface and initiate the proliferation, once the desired atmosphere is achieved. They typically produce an enzyme called adhesins that help the microorganisms to adhere themselves to the host. This stage clearly targets the attachment process between the organism and surfaces. Therefore, in this stage, therapeutic intervention can be performed by completely disrupting the adherence mechanism of the microbes with the surface by targeting the cell surface-associated enzymes called adhesins. An interruption between the microbes and surfaces can be achieved through this method and the initial formation of biofilm can be ceased [
22].
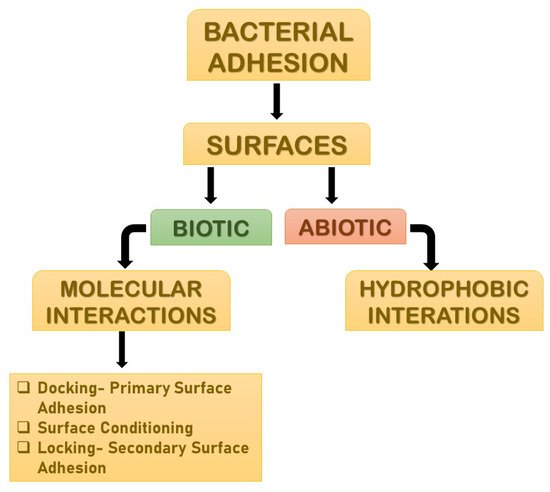
Adhesion is the foremost parameter required for biofilm formation. The initiation and dispersal of the biofilm begins with the adhesion capacity of a particular species in relation to the surface. The use of surface adhesion along with the development of biofilm formation is the survival strategy of the bacteria that usually anchor themselves in a specific way so as to acquire nutrition from the environment. Two distinct phases of adhesion are important before the beginning of biofilm formation (
Figure 3). They are primary or reversible adhesion and secondary or irreversible adhesion. These phases are completely controlled by the different expressions of the genes. The prime rationale behind this feature of surface adherence in bacteria is because the concentration of the nutrients is higher near the solid surfaces. This feature of bacteria plays out in two ways, and either can be beneficial or disastrous. Practically, any sort of surfaces is meant to be the surface for showing the adherence activity of bacteria. The surfaces can be abiotic as well as biotic in nature. The adhesion processes to take place and the number of elements that are taken into consideration include bacterial species, environmental status, the composition of the surface and the gene products. The primary adhesion with the abiotic surfaces occurs with hydrophobic interactions, whereas when the adhesion happens with biotic surfaces, it is mediated by particular molecular interactions. Below,
Figure 3 describes the distinct phases of adhesion [
25,
26].
Figure 3. The distinct phases of adhesions. They are mainly primary or reversible adhesion and secondary or irreversible adhesion. These phases are completely controlled by the different expressions of the genes. The reason behind this feature of surface adherence in bacteria is because the concentration of the nutrients is higher near the solid surfaces. This feature of bacteria plays out in two ways, and either can be beneficial or disastrous.
2.2. Early Biofilm Formation
Early biofilm formation occurs when bacteria begin to divide and create extracellular polymeric substances (EPS), which improves adherence, while also producing the matrix that encases the cell. This characteristic of biofilm can be used as part of a therapeutic intervention by targeting the production of extracellular polymeric substances along with the cellular division of the organism.
2.3. Biofilm Maturation
Biofilm maturation, during which 3D structures form wherein the EPS matrix serves as a multifunctional and protective substratum, allows for the formation of diverse chemical and physical microhabitats in which microorganisms prevail within polymicrobial and social interactions. Physical removal, the destruction of the EPS matrix, targeting the creation of pathogenic microenvironments (low pH or hypoxia) and social interactions (in polymicrobial biofilms), as well as the elimination of dormant cells, might all be used to disrupt the established biofilms that manifest, giving numerous opportunities for therapeutic intervention [
26].
2.4. Dispersal
To convert the sessile biofilm into a motile form, microbial cells begin to disperse in a natural way during this phase. Bacteria that do not generate extracellular polysaccharides, on the other hand, scatter directly into the environment when mechanical force is applied. Microbial communities create several saccharolytic enzymes during dispersion, which release surface microorganisms into a new location for colonization. N-acetyl-heparosan lyase is produced by
E. coli, hyaluronidase is produced by
Streptococcus equisimilis, and alginate lyase is produced by
Pseudomonas aeruginosa and
Pseudomonas fluorescens. Flagella development occurs when microbial cells upregulate protein expression, and the process allows bacteria to travel to a new location, assisting in the spread of diseases. EPS matrix remodelling or the activation of dispersal pathways might cause biofilm dispersion, which can help in controlling the biofilm growth [
25].
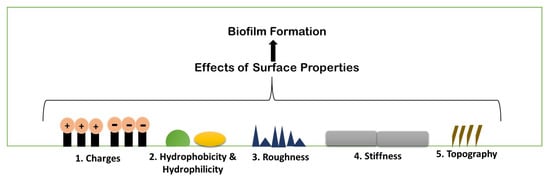
3. Physio-Chemical Surface Properties Controlling Biofilm Formation
Biofilm formation is a strenuous process, which includes specific physiochemical aspects for better formation. A number of parameters are considered for biofilm formation. The figure below describes the parameters briefly (
Figure 4).
Figure 4. Schematic illustration of different existing properties of surface or biofilm formation.
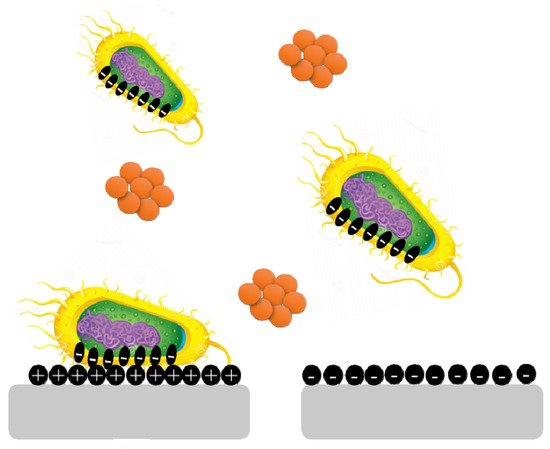
3.1. Surface Charges
Surface charge is known to affect biofilm development and plays a crucial role in defining the attractive and repulsive forces between bacteria and the surface. Because most bacterial cells are negatively charged, a positively charged surface is more susceptible to bacterial adhesion, whereas a negatively charged surface is more resistant.
Pseudomonas aeruginosa biofilms are generated on negatively charged poly(3-sulphopropylmethacrylate) surfaces and contain more cyclic diguanylate monophosphate than biofilms developed on positively charged poly(2-(methacryloyloxy)-ethyl trimethyl ammonium chloride) surfaces (
Figure 5) [
35].
Figure 5. Bacterial cells are negatively charged, and a positively charged surface is more vulnerable to bacterial adherence than a negatively charged surface.
3.2. Hydrophobicity and Hydrophilicity
Bacterial adhesion can be either increased or prevented by adjusting the hydrophobicity of a surface. Biofilm forms less frequently on hydrophobic surfaces than on hydrophilic surfaces. The hydrophobicity of the cell surface is important for adhesion to and dissociation from surfaces. In medicine, bioremediation, and the fermentation industry, cell surface hydrophobicity has both negative and beneficial effects on microbe adhesion to biotic and abiotic surfaces. Surface damage is caused by hydrophobic microbes forming biofilms; on the other hand, they can readily gather on organic contaminants and breakdown them. Microorganisms that are hydrophilic also play a role because of their great resistance to hydrophobic substances, hydrophilic microbes aid in the removal of organic waste from the environment [
36].
3.3. Roughness and Topography
Because of the increased contact area between the material surface and the bacterial cells and the protection from shear pressures, an increase in surface roughness increases bacterial adhesion. However, the precise effects of surface roughness on bacterial adhesion and biofilm formation vary depending on bacterial cell size and shape, as well as other environmental conditions. With increasing surface roughness and better cell adherence to rougher surfaces, adhesion forces rise.
E. coli and
Proteus mirabilis, for example, elongate into filaments, increasing flagella surface density and increasing their flexibility and adhesion potential on rough surfaces [
36].
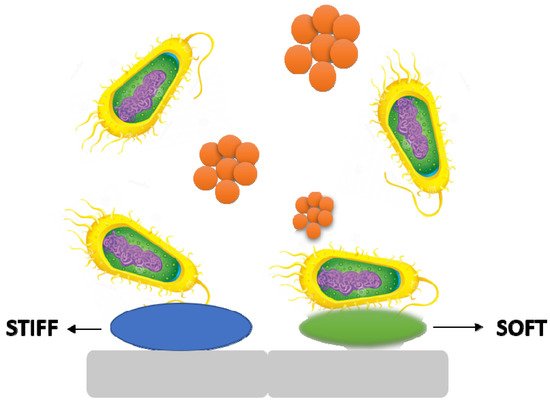
3.4. Stiffness
It is becoming obvious that material stiffness has an impact on bacterial adherence and associated cell function. It is still unclear how surface stiffness and topography (apart from roughness) affect bacterial adherence and biofilm growth (
Figure 6) [
37,
38].
Figure 6. The stiffness of a material has an effect on bacterial adhesion and cell activity.
4. Environmental Conditions
Biofilm formation is largely influenced by the environment, and the processes by which individual cell gene expression influences biofilm development have piqued researchers’ curiosity. Although the mechanisms involved in the process of biofilm formation differ depending on the features of the bacterial strain and environmental conditions, it is now well established that bacteria may cling to practically any surface and create biofilms (
Figure 7).
Figure 7. Several parameters related to environmental conditions for biofilm formation.
5. Emergence of Mixed Biofilm
In nature, most biofilms are generated by numerous microbial species, and so forth mixed-species biofilms mimic microorganisms’ true lives, including bacteria, fungus, viruses (phages), and protozoa. The supply of water, the food service industry, and human and animal health are all threatened by mixed-species biofilm development and environmental resilience. Mixed-species biofilms are thought to be responsible for 60–80% of microbial illnesses. The physiological events that occur during mixed-species community cell proliferation and biofilm development are complicated. Exterior treatments from hostile environments cause high levels of resistance and physiological changes in microorganisms, which are prominent difficulties in biofilm control. Biofilms contain microbial cells incorporated in extracellular polymeric substances (EPS), and they account for roughly 90% of the biofilm volume [
39]. The “one organism—one disease” approach is used in traditional diagnoses and treatment regimens for infections, including chronic wounds. Chronic infections, on the other hand, frequently contain a large number of species in biofilms, but interactions across individual organisms are poorly known. Although the impact of bacterial population and interspecific interactions on the severity and treatment of chronic infections is unknown, recent experimental investigations show that the presence of two or more species increases resistance and virulence when compared to single-species infections. In mixed-species biofilms, cooperative microbial relationships may be based on improving the adherence of the secreted matrix produced by partners or metabolic cross-feeding with products that support the growth of other members [
40]. Microbes in mixed-species biofilms may potentially help to boost resistance. For the physiological contribution of the entire consortium, the arrangement of microorganisms inside mixed-species biofilms is delicately controlled. Mixed-species biofilms can create an intermixing structure, whereas single-species biofilms lack commensal interactions between species. Due to the shift in the makeup of the biofilm matrix and the increased microbial interactions in the consortia, the major mechanisms of higher resistance among mixed-species biofilms are not totally known. Several explanations have been presented to describe the increased resistance in mixed-species biofilms. For instance, certain species may shield others by aggregating with other strains within a three-dimensional structure physically constructed by other species. The thickness of the matrix is thought to have a role in mixed-species resistance in biofilm by preventing risk factors from reaching the active bacteria’s lower levels. Interspecies interactions, such as antimicrobial resistance genomic swap, antimicrobial enzymatic transition, quorum-sensing signal-induced gene expression changes, and metabolite-mediated electron transport chain inhibition, can alter the physiology of neighbouring species in a mixed-species biofilm. These pathways could lead to mixed-species biofilms being more resistant to antibiotics than planktonic cells, as well as the therapeutic challenge of chronic biofilm-associated infections in the host. Various interactions, such as bacteria–bacteria, bacteria–fungus, and bacteria–virus, form the mixed biofilms that are responsible for the chronic infections, or any other biofilm-associated infections, in the hosts [
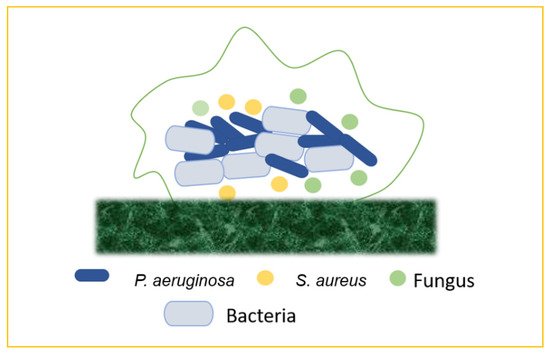
41] (
Figure 8).
Figure 8. Mixed biofilms (bacteria–bacteria; bacteria–fungus; bacteria–virus).
5.1. Bacteria–Bacteria Mixed Biofilms
Biofilm production may result from the bacterial colonization of chronic wounds, which causes local and systemic inflammation and slows the healing process. Multiple bacterial species commonly inhabit chronic wounds, with
S. aureus and
P. aeruginosa being the most prevalent. Lung infections, particularly in cystic fibrosis, are frequently polymicrobial. Periodontal disease is another remarkable example of mixed-biofilm-associated bacterial infection. This term refers to a variety of clinical symptoms ranging from moderate, reversible gingivitis to severe, chronic periodontitis, which can result in the progressive degradation of bone and connective tissue in the periodontal area, as well as tooth loss [
42].
5.2. Bacteria–Fungi Mixed Biofilms
Bacteria and fungi frequently coexist in same ecological and bodily habitats, generating polymicrobial biofilms and developing interkingdom interactions that are progressively being recognized as important in the aetiology of many illnesses. Bacteria–fungi mixed infections can arise anywhere around the body, including the skin, the mouth, the lungs, the gastrointestinal system, the female reproductive tract, and the bloodstream, as a result of the mixed colonization of intravascular devices. When
S. aureus binds in huge numbers to
C. albicans hyphae, the bacteria become more resistant to vancomycin [
43].
5.3. Bacteria–Virus Mixed Biofilms
Emerging research reveals that bacteria and viruses may develop a complex interplay that influences the outcome of mixed infections and the response to antimicrobial/antiviral therapy. Although bacteria do not allow eukaryotic viruses to infect them, they can aid viral fitness by improving virion stability, encouraging eukaryotic cell infection, or raising coinfection rates. Viruses that bind to bacteria, on the other hand, may enhance bacterial adhesion to eukaryotic cells. For example, in cystic fibrosis patients,
P. aeruginosa and
respiratory syncytial virus (RSV) are the two most common infections [
44].
6. Biofilm-Associated Human Diseases
Biofilms are becoming more widely acknowledged as a factor in human disease. According to studies, 24% of adults have lost at least 4 mm of periodontal attachment, and 40–50% of adults, 60% of 15-year-olds have some sort of gingival (biofilm) infection. Biofilms are also key colonizers of medical equipment, such as catheters, shunts, and venous and arterial shunts. Thus, 15–20 percent of 4000 newborns with cerebrospinal fluid shunts were found to be infected by a biofilm in a study. Respirators, sigmoidoscopes, contact lenses, and artificial implants (such as heart valves, pacemakers, ventricular assist devices, synthetic vascular grafts, and stents) are also included. Biofilms have been found on urinary prosthesis as well as orthopaedic prostheses (such as artificial joints). The majority of untreated chronic diseases in humans are assumed to be of biofilm origin by the biofilm research community. Human biofilm infections are caused by two different properties shared by all biofilms. To begin with, biofilms are extremely resistant to immunological destruction and clearing, as well as antimicrobial treatment. Second, biofilms that are well-protected may be capable of shedding individual bacteria and sloughing biofilm fragments into neighbouring tissues and the circulatory system. These shed cells could be the source of an acute sickness that recurs after antibiotic treatment [
36].
Importantly, biofilm formation as a defensive measure could have far-reaching consequences for the host, as bacteria thriving in these matrix-enclosed aggregates are more resistant to drugs and host defences. Microbial cells developing in biofilms are said to be clustered, according to the biofilm model. This casts doubt on the idea that infectious pathogens are evenly dispersed and, thus, equally susceptible to the host immune system or antibiotic therapy. It could also explain a number of difficult clinical issues, including symptomatic but unculturable inflammation, antibiotic resistance, recurrence or persistence, and metastasis or dissemination. Despite the fact that intravenous catheters, prosthetic heart valves, joint prostheses, peritoneal dialysis catheters, cardiac pacemakers, cerebrospinal fluid shunts, and endotracheal tubes save millions of lives, they all carry the risk of surface-associated infections. The
Staphylococci (especially
S. epidermidis and
S. aureus) are the bacteria most frequently associated with medical devices, followed by
P. aeruginosa and a slew of other environmental bacteria that unscrupulously infect a host who is compromised by invasive medical intervention, chemotherapy, or a pre-existing disease state. The development of biofilms on medical implants has even resulted in the identification of a novel infectious condition known as chronic polymer-associated infection [
45,
46].
7. Therapeutic Approaches to Combat Biofilm Development
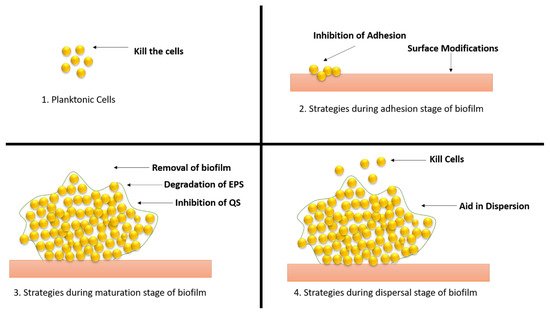
Biofilm-related infections and the obstacles associated with their treatment have been identified as important dangers to human health in recent years, prompting the development of solutions to address the issue (
Figure 9).
Figure 9. Different strategies of therapeutic approaches to combat the biofilm development. (1) Strategies applied to planktonic cells; (2) approaches applied during adhesion stage; (3) approaches applied during maturation stage; (4) approaches applied during dispersal stage.
CD437 is a new synthetic retinoid antibiotic that kills methicillin-resistant S. aureus (MRSA) persister cells by disrupting their lipid bilayer, and it has an excellent synergistic antibacterial activity with gentamicin. Similarly, 12 amino acid sequences were inserted into tobramycin, an aminoglycoside antibiotic, to create Pentobra, a modified antibiotic. Researchers attempted to integrate several methods of antibacterial action by imitating the function of antimicrobial peptides with the connected amino acid sequences. E. coli and S. aureus were both resistant to the complex antibiotic. More molecules might enter persister cells and trigger bactericidal action because the polypeptide increased cell membrane permeability. Because there are no specific protein targets and bacteria are unlikely to acquire drug resistance, host defence peptides are good options for treating dormant persister cells. Peptides have low in vivo stability, involve a time-consuming manufacturing process, and are expensive, despite the fact that some peptides exhibit significant antibacterial activities. All of these factors limit the use of host defence peptides in therapeutic settings (they are also called antimicrobial peptides). Researchers have been focusing on producing peptide mimics, particularly polymeric mimics that can be made quickly and cheaply, in light of these factors. The mimics have good antibacterial effects because their structural qualities are similar to those of host defence peptides. Poly(2-oxazoline)s, which were recently discovered, can act as a new class of functional peptide mimics, demonstrating that poly(2-oxazoline)s can perform as powerful antibacterial effects as the synthetic mimics of host defence peptides. The discovery of peptide mimics opens up new possibilities for antibacterial drug development [
48,
49].
Mass sensing is a microorganism-to-microorganism communication system that regulates the expression of genes by reacting to the density of the population and the proximity of other microbes.
When small chemicals called self-inducers or quorum-sensing molecules aggregate during cell development, they cause changes in microbial gene expression. Gram-negative bacteria use N-acyl homoserine lactones (3-oxo-dodecanoyl- and butanoyl-HSL) and the quinolone signal (2-heptyl-3hydroxy-4(1H)-quinolone) as intra-species signalling molecules. In the extracellular environment, acylase can cleave the amide bond of acyl-homoserine lactones (AHLs), a quorum-sensing signal released by Gram-negative bacteria. EPS substrate proteins and adhesins can be hydrolysed by proteases. The carrier is a protease-functionalized nanogel. The ciprofloxacin encapsulation inside the nanocarrier dispersed the S. aureus biofilms and decreased the viability of bacterial cells within the biofilms.
Natural compounds have been demonstrated to have antibacterial and anti-biofilm activity against
P. aeruginosa, including a metabolite generated by the endophytic bacterium
Streptomyces ansochromogenes. Maipomycin A (MaiA) is a new antibiofilm chemical isolated using a bio-guided assay from
Kibdelosporangium phytohabitans XY-R10. MaiA is a promising option in the fight against Gram-negative biofilms. Water-borne illnesses are caused by pathogens that can be found in planktonic form or as biofilms in water-carrying pipelines. Marine mussel protein-based adhesives, which include the catecholic amino acid 3,4-dihydroxyphenylalanine (DOPA), may stick to nearly any substrate. Polydopamine (PDA), a new catechol derivative and dopamine-based coating material, has also been developed [
53].
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics11040476