2. SARS-CoV-2 Structure and Infection Mechanism
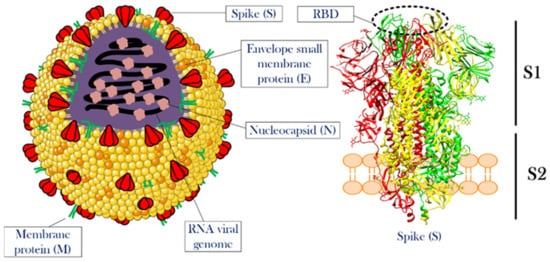
Knowing the structural features and life cycle peculiarities of the virus will enable researchers to suggest solutions to deal with the virus outbreak. With their genomes approaching 30 kb in length, CoVs are among the largest known RNA viruses. SARS-CoV-2 is an enveloped positive-strand single-strand RNA virus (+ssRNA virus), whose genomic ssRNA is condensed by the nucleocapsid (N) proteins at the center of the viral particle. The size of the viral particle of SARS-CoV-2 can be up to 100 nm [
21]. The outermost layer of the viral particle is made of a phospholipid membrane similar to mammalian cells, which contains three types of viral proteins. These proteins include membrane (M) protein in high abundance, coating proteins (envelop protein, E protein) in relatively low abundance, and most importantly, spike protein (S protein) [
22,
23]. S protein is a trimeric glycoprotein, a monomer that has a total length of 1273 amino acids. There is a 76% sequence similarity between the S proteins of SARS-CoV and SARS-CoV-2, and they are glycosylated at 21 to 35 sites, respectively.
The S protein consists of two main parts called S1 and S2 subunits. The S1 subunit has a segment that detects mammalian cellular receptors (receptor-binding domain, RBD) and is responsible for binding the viral particle to the host cell, while the S2 subunit has trans-membrane domains that hold the protein like a rod into the viral membrane [
21,
24]. Thanks to the structural studies of this protein, it was observed that as soon as S protein binds to receptors on the surface of the host cell, the open structure of this protein becomes closed. This structural switch ensures a strong attachment between the virus and the host cell.
M and E proteins play structural roles for viral particles [
25,
26,
27].
Figure 1 illustrates the overall view of a CoV particle. Attachment to the surface of the host cell followed by cell entry is among the most important aspects of the life cycle [
28]. A correct and in-depth understanding of this stage of viral infection can help in designing drugs to prevent the virus entrance into the host cell. Although this stage of the virus’ life cycle has not yet been completely uncovered, significant progress has been made in this field. In this article, a detailed description of the stages of viral infection will be presented, and the mechanisms that have been proposed to fight the virus at each step will be discussed.
Figure 1. The overall structure of CoV and the spike protein. The left side illustrates a single particle of CoV. Three membrane proteins and RNA viral genome in a complex with nucleocapsid proteins were shown in the scheme. The right side shows the detailed structure of a spike protein. This protein, which is the most important functional protein during the attachment of the viral particle to the host cell, has two subunits named S1 and S2. The receptor-binding domain is located at the top of S1. For more information see the text. The structure of the spike protein was extracted from PDB 6ZGI [
29]. RBD is receptor-binding domain.
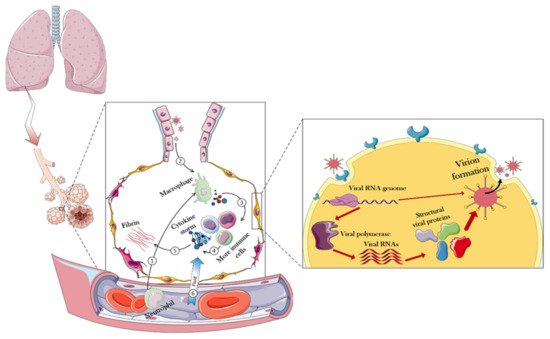
To design a safe drug, one has to understand the effects of the target virus on the body. Figure 2 presents details of how the body responds to the virus and shows that the virus first enters the lungs through the respiratory system, and then the immune system in the alveoli would be used as the first level of defense against the virus.
Figure 2. Immune responses and overall intracellular events triggered in the face of CoV-2. In normal conditions, immune cells enter the alveoli of the lungs through the blood. When CoV arrives in the alveolar compartment, these immune cells oppose them and eventually produce cytokines. Positively regulating, cytokines trigger more immune cells, eventually producing more cytokines. During these events, the fibrin of the alveoli increases, resulting in partial destruction and increased permeability of the alveoli. Consequently, fluid goes on the battlefield (alveoli) through the capillaries, causing destruction and edema of the infected lung. However, when a virus can compete with these challenges, it will be able to enter the host cell. In the host cell, the viral genome is generally released and the host equipment is used to replicate the viral particles. The following sections of the article provide more details in this regard.
To that end, immune cells that have entered through blood vessels into the alveoli, secrete a variety of cytokines in contact with the invading virus [
30]. This response, by itself, triggers more immune cells that would generate more cytokines, resulting in a cytokine storm [
31]. Among the most important cytokines secreted at this stage are the interleukins 6 and 1 (IL-6 and IL-1), as well as α-interferon (INF-α). The total secretion of cytokines causes the overproduction and exudation of fibrin in the alveoli, which, by disruption of cell junction, can eventually increase the flow of blood fluid through the capillaries to the lung chamber, resulting in pulmonary edema, focal hemorrhage, and pulmonary consolidation [
32,
33,
34,
35]. All of this occurs due to the body’s intense inflammatory responses against the invading virus [
36].
Upon closer inspection of the infected cell, the first step in the virus entering the host cell is the interaction between the S protein (RBD of the S1 subunit) and the angiotensin-converting enzyme 2 (ACE2) receptor at the surface of the host cell [
37,
38,
39]. The analog for this receptor in the MERS-CoV infection mechanism is dipeptidyl peptidase-4 (DPP4) receptor [
40,
41]. The angiotensin converting-enzyme is an important membrane protein that is abundantly expressed on the surface of cells of various human and animal tissues. The tissues with the highest expression levels of ACE2 are the lungs, gastrointestinal tract, blood vessels, kidneys, liver, and heart. The history of recognizing the importance of this protein dates back to three decades ago [
42].
During these years, scientists speculated on many functions for this protein, including its crucial role in the renin–angiotensin–aldosterone system (RAAS) pathway regulating blood pressure, wound healing, and inflammation. Here, ACE2 modulates activities of angiotensin II (ANG II), a protein that increases blood pressure, body water and sodium content, and inflammation, as well as increases damage to blood vessel linings and various types of tissue injury, by converting ANG II to other molecules that counteract the effects of ANG II. Concerning the molecular mechanisms of CoV-2 infection, ACE2 serves as a major receptor controlling the main route of SARS-CoV-2 entry to the host cells [
43].
The importance of this receptor is further emphasized by finding a close relationship between COVID-19 vulnerability and gastrointestinal symptoms due to high expression levels of the ACE2 receptor at the gastrointestinal epithelial cells [
44,
45]. However, some researchers have suggested that SARS-CoV-2 may also enter the host cell through interaction with another receptor, the CD147 protein [
46], although this claim has been questioned in more recent studies [
47]. This receptor is also expressed on the surface of many tissues and is responsible for changing the shape of the matrix in some phenomena, such as cancer, inflammation, and wound healing [
48]. It is believed that the reason for the different responses of different patients to viral infection is a difference in the expression level of these receptors at the surface of their cells. With all this, as soon as the interaction between ACE2 and S protein has taken place, the viral particle enters the host cell by endocytosis, and the virus genome is released [
49]. However, one should take a closer look at the roles of another protein in this process. The binding of S protein to ACE2 is not sufficient for cell entry, and the internalization of the virus particle to the host cell is activated by the specific cleavage of the ACE2-bound S protein by a transmembrane serine protease protein-2 (TMPSS2) [
50,
51]. As another serendipity of the human body, this protein has been closely linked to many health problems. For example, the crucial roles of TMPSS2 in the metastasis and progression of prostate cancer are well-known [
52].
After entering the cell, due to the suitable conditions for viral protein translation, the host translational machinery starts producing viral RNA-dependent RNA polymerase, and this event marks the beginning of a cascade of intracellular processes in favor of the invading virus. After the viral polymerase is translated, a variety of viral RNA genes is produced, resulting in the expression of structural and functional proteins needed to form a complete viral particle inside the host cell [
53,
54]. Consequently, at the last stage of viral infection, whole viral particles are expelled by the explosion of the host cell or exocytosis, infecting the surrounding healthy cells. All of these pathways have been used to design strategies to combat the SARS-CoV-2 infection [
55,
56,
57].
3. Nanomaterials and the Development of CoV-2 Vaccines
Nanomaterials have been used in various fields for decades. This group of materials in general, with their very large surface-to-volume ratios, the ability to place molecules with different properties on their surface (functionalization), and simplicity in their production process have played a major role in advancing human knowledge and increasing quality of life [
58,
59,
60]. Nonetheless, although some scientists have discovered new usages for the substances, others pointed to their side effects. Thereby, this type of material should be considered a double-edged sword. In this section, different applications of nanomaterials in the inhibition of the virus life cycle are presented.
3.1. Different Classes of Nanomaterials against Coronavirus Disease
This ongoing pandemic, with all its bitterness, proved that the harmony between the sciences could be a great help in achieving human goals. For example, it was observed that the use of various nanoparticles along with molecular biology eventually introduced vaccines into the consumer markets, which turned the dark days of the epidemic into a beacon of hope. Scientists in the nanotechnology field have followed various strategies, each with a vision to generate means to fight the virus. Some nanoparticles have been used to directly fight the virus [
61], while others have been used for the rapid detection of viruses in laboratory samples [
62]. Additionally, some nanocarriers are used to deliver anti-SARS-Cov-2 drugs/vaccines [
63].
To deal with global epidemics, careful review and utilization of all available tools/means are important. In this regard, the use of nanotechnology as a new field in medical sciences and its multifunctional structures can be a solution. Nanotechnology can be used for a variety of medical purposes, such as clinical diagnosis, pharmaceutical research, immune system activation, and the extraction of the biological materials. To defeat COVID-19, better understanding of the virus, better diagnosis of infection, its treatment and prevention are steps in which nanotechnology is expected to help [
64].
Polymer nanoparticles have found their place in many industrial and medical fields. These substances, especially in regenerative medicine, have been able to give much hope to the patient community to recover from tissue degeneration diseases [
65]. Having a high level of safety, biodegradability, simplicity of synthesis, and the ability to control their properties through different functionalizations initiated strong attention to nanoparticles in the field of anti-SARS-CoV-2 research [
61]. Another positive point for this group of nanoparticles is that some of them have been approved by the Food and Drug Administration (FDA) and have been studied in great detail in other fields [
66]. By selecting this group of nanoparticles, the distance to reach the final goal may not be as long as the one needed for the development of novel and completely unknown nanoparticles.
Certainly, the synthesis methods of these nanoparticles, which directly affect their properties, will also indirectly determine the effectiveness of nanoparticles in combating the new coronavirus. The sizes of nanoparticles, which are generally between 1 and 100 nanometers, guarantee a very high surface to volume ratio, as well as the ability to load significant amounts of drugs in small amounts of nanoparticles, which can help a lot in combating pathogens, including SARS-CoV-2 virus [
67].
3.2. Antiviral Mechanism of Nanoparticles
The antiviral mechanisms of nanoparticles include inhibiting the binding of the virus to the target cell, preventing the virus from entering into the host cell, and attacking the growth and proliferation stage of the virus. Possible mechanisms of nanoparticles include direct and indirect inactivation of viruses. These mechanisms vary depending on the three-dimensional shape and type of nanoparticles [
68,
69]. Another mechanism proposed for the antiviral action of nanoparticles is the local field action of nanoparticles. In this way, the designed nanoparticles change the membrane potential at the surface of the host cell as soon as they are adsorbed on the cell surface. Following this, membrane potential change and the penetration of the virus into the host cell are affected and reduced [
70].
Other studies have suggested that metal nanoparticles, such as those containing silver ions with oxidizing properties in infected host cells, can prevent the virus from spreading to the healthy cells [
71]. According to an in silico study, iron nanoparticles have also been shown to form a stable complex with the CoV spike protein and prevent the virus from attaching to the host cell [
72].
Table 1 lists some of the studies that used nanoparticles in fighting respiratory viral diseases [
73].
Table 1. Previously used different classes of nanoparticles in respiratory viral diseases.
Abbreviations: RSV, respiratory syncytial virus; TLR, toll-like receptor; TH1, T helper type 1; PLGA, Poly(d,l-lactide-co-glycolide).
3.3. Properties of Nanoparticles for Efficient Vaccine Production
Vaccines have shown great potential for use in the prevention and treatment of infectious diseases. With the rapid development of biotechnology and materials science, nanomaterials have found an essential place in the formulation of new vaccines, as they can enhance the effect of antigens by acting as a release system and/or as an immune-boosting aid [
74]. The analysis of the effects of nanoparticles on vaccine properties shows the improvement of the antigen stability and immunogenicity as well as a capability of targeted and controlled release of active substances [
74]. However, there are still obstacles in this field due to the lack of fundamental knowledge on how nanoparticles act at the molecular scale, and what the biological effects of nanoparticles in living organisms are [
75]. Nanoparticle-based vaccines are classified based on the function of the nanoparticles in them as a release system and immune response enhancer. Therefore, a fundamental understanding of the distribution of nanoparticles in the body and their fate will accelerate the logical design of new nanoparticles that will change the future of vaccines. Nanotechnology has provided the opportunity to design different nanoparticles in terms of composition, size, shape, and surface properties for various pharmaceutical applications [
76].
Nanoparticles with the same size as cellular components can show biophysical function and biotherapy similar to their biological counterparts. There are several systematic studies which showed that the nanoparticles designed with polyethylene glycol (PEG) are able to delay the clearance of the drug from the body and thus make the systematic circulation of the drug in the body longer than in the free drug state [
77]. This can eventually be useful for the accumulation of more drug at the site of treatment. In addition, nanoparticle delivery systems can have several salient features, including high drug loading capacity, controlled release rate, and reduced drug toxicity in the body [
78]. As a result, nanoparticle-based approaches as release systems provide new opportunities to enhance innate immune activation and induce a strong immune response to the slightest toxicity [
75]. The most important components of an effective vaccine include an antigen to activate the immune system, an enhancer of the immune response to stimulate the innate immune system, and a release system to ensure proper antigen delivery and targeting [
79]. To achieve these goals, the design of nanoparticles focuses on the chemical composition, size, surface charge, and surface properties of the nanoparticles, as these are used to control the distribution of these particles in the environment, the release of antigen, the efficiency of immune stimulation, and the final immune response [
80]. Emulsions, liposomes, and synthetic polymers are nanoparticles that serve as helpers for the proper release of immune response enhancers. Antigen-carrying nanoparticles are able to affect the immune response and significantly enhance the T-cell cytotoxic response against the antigen fused to the nanoparticles [
81]. This is due to the specialized ability of some antigen-presenting cells (APCs), which can effectively absorb foreign particles, such as microparticles and bacteria [
82]. This process is performed by detecting antigenic material to analyze and express foreign antigens to other cells in the immune system. However, there are limitations to the utilization of these approaches, such as the existence of the nonspecific uptake and immunosuppression activities of these compounds.
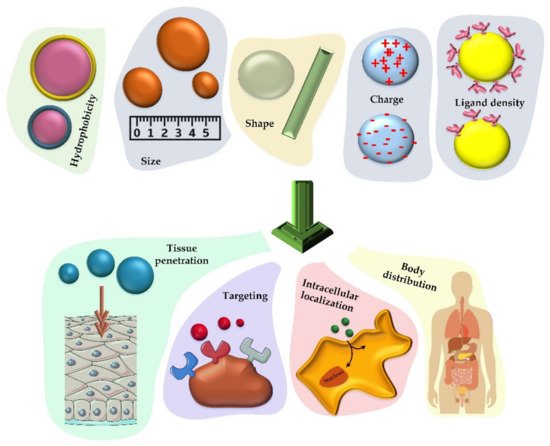
As mentioned, controlling the size, shape, and chemical properties of nanoparticles enables these tiny particles to have a controllable cell uptake coefficient. In order to provide organized information for efficient vaccine production,
Figure 3 and the discussion below indicate the properties that nanoparticles must have to be considered [
80].
Figure 3. Various chemical and physical properties that can determine how nanoparticles will act as vaccine carriers. The size, hydrophobicity, charge, shape, and ligand density of the nanoparticles that carry the therapeutic factor will have a significant effect on their cellular uptake, distribution in the body, accumulation in the cell (especially phagocytic cells) and the rate of tissue penetration.
3.4. Different Nanoparticle-Based Vaccines for CoVs
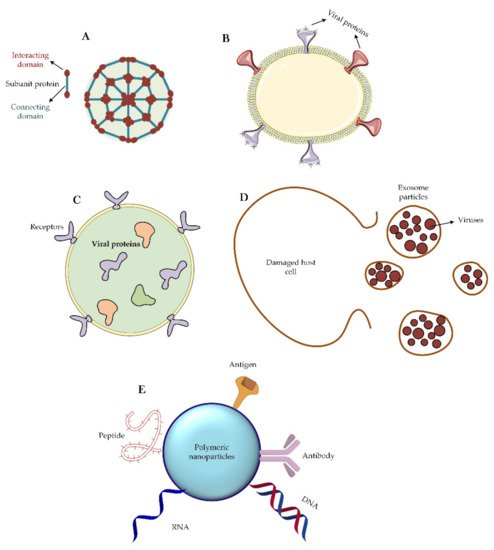
From a structural viewpoint, four types of nanoparticles have been introduced for this purpose (
Figure 4). The first group of these nanoparticles originates from proteins that can self-assemble; e.g., viral proteins that can aggregate into virus-like particles or form protein micelles [
94,
95,
96]. Similar to a virus particle, there is another group of nanoparticles that are liposomes along with the capsid proteins. Liposomes themselves can also be considered as nanoparticles capable of carrying therapeutic agents inside their body against CoV. Finally, exosomes are another group of nanoparticles that are very similar to viruses, except that these particles are usually produced by exocytosis from virus-infected cells [
97,
98].
Figure 4. Different classes of nanoparticles are used as virus vaccines. (A) Self-assembling capsid protein nanoparticle. This type of nanoparticle is made up entirely of proteins that are able to self-aggregate. Sometimes two or three types of proteins are used to make this nanoparticle. (B) Virus-like particle. Particle engineering has created the ability to design and synthesize a virus-like particle that is an assembly of a phospholipid and a set of viral proteins. (C) Liposome. Liposomes are free of any viral proteins on their surface. Sometimes they may have receptors for the correct targeting of the particle, but it should be noted that viral proteins are trapped inside the liposome structure and enter the immunological pathways into the host cell after the endocytosis of the particle. (D) Exosome particle. Once the host cell is infected with the virus, an exosome will emerge from the damaged cell that contain the newly synthesized viruses. These particles, after extraction and purification, can be suitable treatment options. (E) Corresponds many available nano-based polymeric materials that functionalized with different therapeutic agents such as DNA, RNA, antigens, peptides, and antibodies.
Today, perhaps the most important application of nanoparticles due to the global CoV-2 challenge is the use of these materials to load and transport viral antigens and viral DNA or RNA genomes [
99,
100]. In the meantime, physical and chemical interactions, such as adsorption, entrapment, and attachment have been used to load viral materials into the nanoparticles. For this important purpose, a variety of nanoparticles, such as nanopolymers [
101], liposomes [
102,
103], and quantum dots [
104,
105] have been used [
100]. Between the years 2014–2018, scientists used protein micelles consisting of the S protein of SARS-CoV-1 and MERS-CoV to fight these viruses [
106,
107]. Of these, some remain in the early clinical stages, but recently, this method has also been used to deal with the SARS-CoV-2 infection.
The vaccine-related virus-mimicking nanoparticles (NPs) such as self-assembled viral proteins and virus-like particles are in phase I clinical trials [
97]. The advantages of using this group of nanoparticles include simplicity of their production, safety, high resistance in vitro, and virus-like body distribution. However, this type of nanoparticle also has some disadvantages, such as high production cost, the difficulty of industrialization, low stability in vivo, and occurrence of unwanted immune reactions [
97]. Studies to suppress the progression of MERS-CoV [
108,
109] and SARS-CoV-2 [
97] have been performed using virus-like particles of S proteins and RBD domains, respectively. Both of these approaches are in the early clinical phase.
In the case of the liposomes, the most important challenge is the limited cargo capacity and the fast release of the cargo to the environment. However, these types of nanoparticles have several advantages, such as relative easiness of production, long-term physical stability, and high control over surface properties. These nanoparticles, in conjunction with S and R protein-encoding RNAs, have also been used to control the progression of CoVs such as SARS-CoV [
114] and SARS-CoV-2 [
115].
Two types of methods (mechanical and non-mechanical) are used to make liposomes and nanoliposomes. The mechanical methods include sonication, homogenization, extrusion, and microfluidization. The non-mechanical methods are reverse-phase evaporation, discharge of lipid–detergent micelle combination, freeze drying, solvent injection, thin layer dehydration, and the thermal method. The sonication method, the most common method in the production of liposomes, uses sound energy to create cavities and disperse particles [
116]. The cavities created by the sound effect cause the gas bubbles in the liquid to expand and contract. As the waves increase, the bubbles begin to oscillate and eventually burst. As a result, small vesicles form. This method is used in the preparation of monolayer nanoliposomes. This method is also divided into three types of sonication with a probe, sonication in a water bath, and ultrasonication [
117].
3.5. Delivery Role of Nanoparticles: Focusing on the CoVs
Relying on human knowledge, which came from the use of DNA and RNA vaccines, the idea of using nanoparticles for vaccination was introduced in the field of COVID-19 treatment. The use of this strategy to deliver small interfering RNA (siRNA) vaccines has helped control the symptoms of several diseases, such as autoimmune and neurological diseases [
123]. The fundamentals of the preparation of mRNA-1273 vaccine have been based on this idea. The vaccine comprises an RNA genome covered with a lipid-based nanoparticle envelop [
124]. Nanoparticles can also be useful in the delivery of therapeutic antigens. Thanks to the development of science in this field, nanoparticles can be classified into two major types by considering whether the antigen is located inside (encapsulated antigen) or on the surface (surface-presented antigen) of the nanocarrier particle (
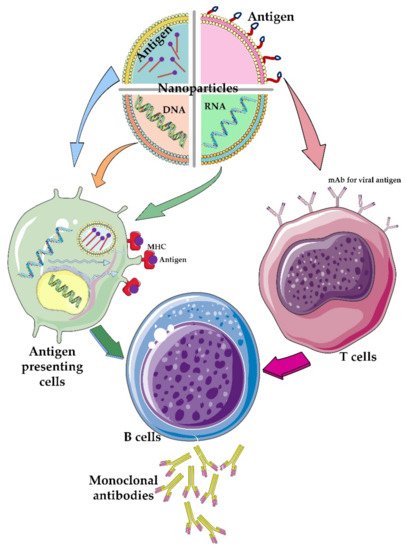
Figure 5).
Figure 5. The role of different nanoparticles containing therapeutic agents. Different nanoparticles have been used to carry different components of viral particles such as genetic material and/or its antigens. The viral genetic materials are usually encapsulated or trapped inside the nanoparticles while the viral antigens are functionalized on the surface of the nanoparticles. Depending on which type of T-cell or antigen-presenting cells these engineered nanoparticles attach to, different immunological pathways are created in the body, which ultimately lead to the activation of B cells that produce monoclonal antibodies against the virus particle.
3.6. Attacking the CoV-2 Life-Cycle with the Help of Nanoparticles
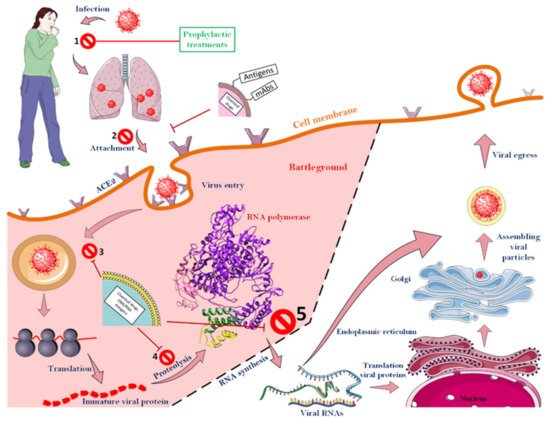
Luckily for us, various steps must be taken to finally create a new virus particle inside a host cell. As illustrated in Figure 6, which shows all the steps of the CoV-2 life cycle, scientists have been able to design various strategies to combat the virus by specifically attacking it at different stages (up to stage 5). To that end, according to the results obtained so far, it is clear that most success in the struggle between the therapeutic agents and the virus can be achieved before the extremely important viral enzyme, RNA-dependent RNA polymerase, starts to function. If the virus cycle cannot be controlled up to this stage, due to the speed of enzyme action and its specific activity and high turnover number of an enzyme, a significant number of viral RNAs will be formed in a short time, and the next steps will proceed at a worrying rate. After this stage, there is almost no hope for blocking the virus life cycle.
Figure 6. Different stages of a CoV-2 life cycle along with target points for fighting the virus. The CoVs can be fought almost from the beginning of the virus to the function of the RNA-dependent RNA polymerase. However, after the function of the enzyme, no specific strategy has been proposed to deal with the virus. As soon as the virus enters the body, a range of events will occur, though up to stage, 5 the virus can still be defeated. Nanoparticles that have monoclonal antibodies or antigens on their surface usually act before the virus enters the cell. The ACE2 receptor, which is among the most important cell surface receptors for the SARS-CoV-2, was a target of many studies for blocking virus cell entry. As soon as the virus binds to this receptor, the process of virus penetration into the host cell begins. Consequently, masking this agent on the surface of host cells can prevent the virus from entering the cell. Nanoparticles that encapsulated therapeutic agents will be able to fight the virus as it enters the host cell. See the text for more details.