Lung cancer is a highly aggressive neoplasm that is now a leading cause of cancer death worldwide. One of the major approaches for killing cancer cells is related with activation of apoptotic cell death with anti-cancer drugs. Regarding lung-cancer therapy, graphene was already considered as an attractive material for screening (biosensing) or direct anti-cancer treatment.
- flake graphene
- graphene oxide
- ferroptosis
- non-small lung cancer
- nanomaterials
1. Non-Small Lung Cancer
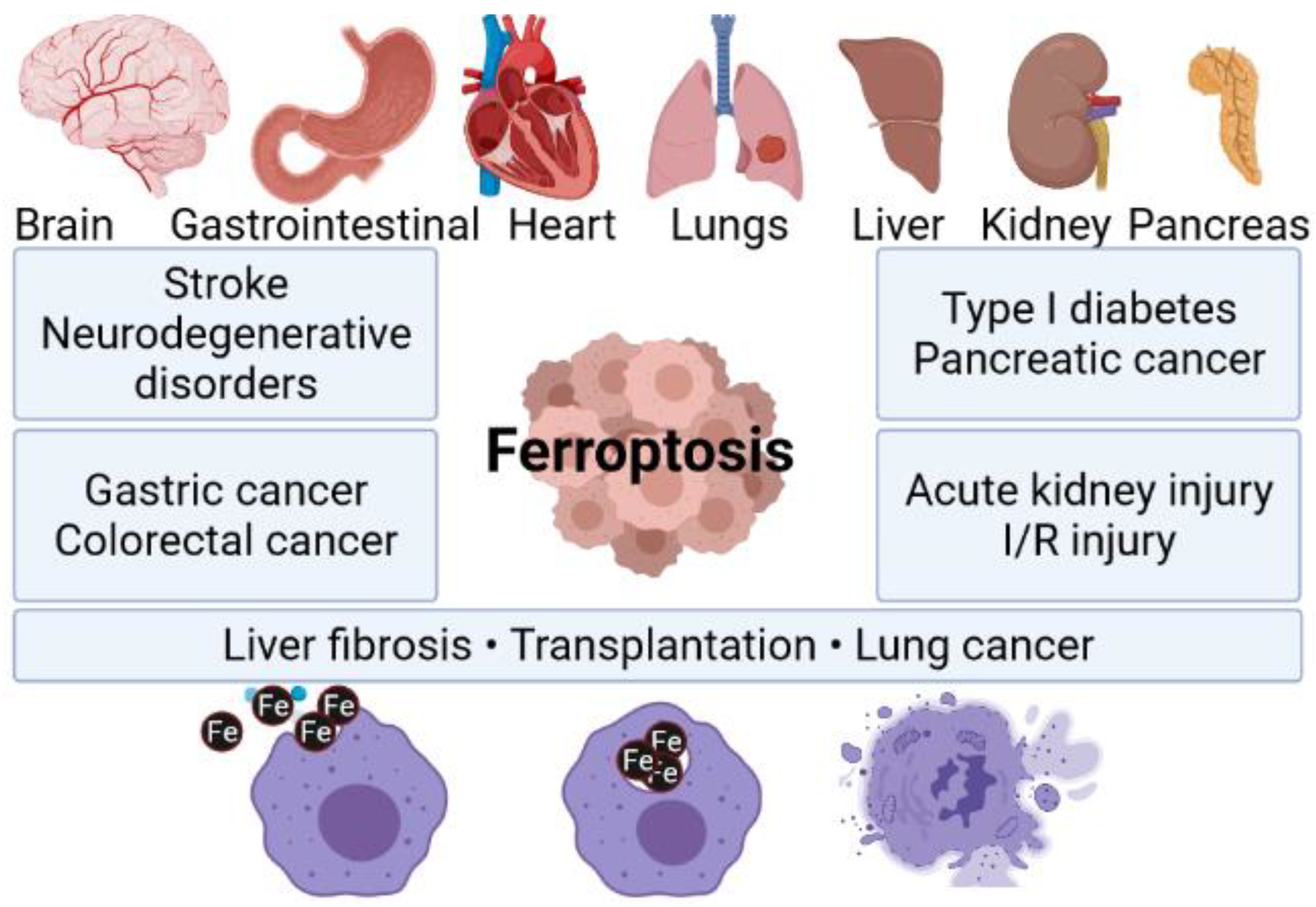
2. Ferroptosis
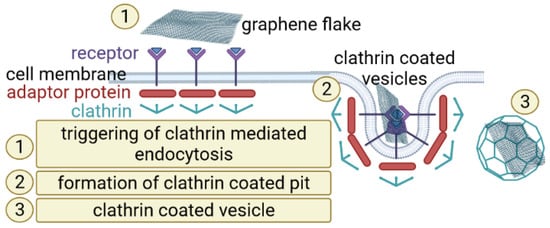
3. Nanomaterial Based Ferroptosis Inducers in NSCLC
|
Nanoparticle Type |
Nanoparticle Size |
Cancer Model |
References |
|---|---|---|---|
|
AIEgen/vermiculite nanohybrid |
300 nm diameter, 1.1 nm thickness |
MC38 tumor model |
[34] |
|
polyvinyl pyrrolidone dispersed nanoscale metal-organic framework of Fe-TCPP (TCPP = tetrakis (4-carboxyphenyl) porphyrin) loaded with hypoxia-activable prodrug tirapazamine and coated by the cancer cell membrane |
201 nm diameter of the whole system |
Breast cancer cells (MDA-MB-231), human liver cancer cells (Huh7, HepG2), human colon cancer cells (HCT116), human pancreatic cancer cells (PATU8988), cervical cancer cells (HeLa) |
[35] |
|
Glycyrrhetinic acid loaded PLGA nanoparticles |
133 nm in diameter |
Leukemia cells: Kasumi-1, U937, MV4–11, NB4, and colorectal cancer cells: HT29, Caco-2, SW480 |
[36] |
|
Gallic acid-ferrous (GA-Fe(II)) |
average particle size of 3.1 ± 1.2 nm and hydrodynamic diameter of 4.7 ± 1.1 nm |
breast cancer cell (MCF-7/ADR) |
[37] |
|
Zinc oxide nanospheres |
120 nm in diameter |
CT26 and HCT116 colorectal cancer cells |
[38] |
|
piperlongumine (PL) loaded metal–organic framework (MOF) coated with transferrin decorated pH sensitive lipid layer |
hydrodynamic radius of 185 ± 5.7 nm |
4T1 brest cancer cells |
[39] |
|
silver coated zero-valent-iron nanoparticles (ZVI@Ag) and carboxymethylcellulose coated zero-valent-iron nanoparticles (ZVI@CMC) |
mean physical diameters of ZVI@Ag NPs and ZVI@CMC NPs were 81.08 ± 14.29 nm and 70.17 ± 14.4 nm |
Human lung cancer cell lines H1299, H460, A549, mouse Lewis lung carcinoma (LLC) |
[40] |
|
Fe(II) and Tannic Acid-Cloaked MOF |
125–225 nm |
MDA-MB-231 epithelial, human breast cancer cell line |
[41] |
|
Manganese doped silica nanoparticle (MnMSN) and folate modified long-circulating MnMSN (FaPEG-MnMSN) |
MnMSN—diameter of 101.40 ± 0.36 nm, FaPEG-MnMSN diameter of 122.67 ± 2.98 nm |
human hepatic carcinoma cells (HepG2), human non-small lung cancer cells (A549) and mouse breast cancer cells (4T1) |
[42] |
|
Superparamagnetic iron oxide nanoparticles (SPION) |
99–115 nm of hydrodynamic dimeter |
Mouse mammary breast tumor cell line (4T1), human breast cancer cell line (MDA-MB-231) and human breast cancer cell line (MCF-7) |
[43] |
This entry is adapted from the peer-reviewed paper 10.3390/ma15103456
References
- Pikor, L.A.; Ramnarine, V.R.; Lam, S.; Lam, W.L. Genetic Alterations Defining NSCLC Subtypes and Their Therapeutic Implications. Lung Cancer 2013, 82, 179–189.
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8.
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386.
- Shin, A.; Oh, C.M.; Kim, B.W.; Woo, H.; Won, Y.J.; Lee, J.S. Lung Cancer Epidemiology in Korea. Cancer Res. Treat. 2017, 49, 616–626.
- Iglesias, V.S.; Giuranno, L.; Dubois, L.J.; Theys, J.; Vooijs, M. Drug Resistance in Non-Small Cell Lung Cancer: A Potential for NOTCH Targeting? Front. Oncol. 2018, 8, 267.
- Lee, J.S.; Hong, J.H.; Sun, D.S.; Won, H.S.; Kim, Y.H.; Ahn, M.S.; Kang, S.Y.; Lee, H.W.; Ko, Y.H. The Impact of Systemic Treatment on Brain Metastasis in Patients with Non-Small-Cell Lung Cancer: A Retrospective Nationwide Population-Based Cohort Study. Sci. Rep. 2019, 9, 18689.
- Yoon, S.M.; Shaikh, T.; Hallman, M. Therapeutic Management Options for Stage III Non-Small Cell Lung Cancer. World J. Clin. Oncol. 2017, 8, 1–20.
- Geiger, S.; Schlemmer, M.; Heinemann, V.; Stemmler, H.J. Adjuvant Cisplatin-Based Chemotherapy for Resected NSCLC: One Size Fits All? Anti-Cancer Drugs 2010, 21, 799–804.
- Jiang, W.; Cai, G.; Hu, P.C.; Wang, Y. Personalized Medicine in Non-Small Cell Lung Cancer: A Review from a Pharmacogenomics Perspective. Acta Pharm. Sin. B 2018, 8, 530–538.
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer Drug Resistance: An Evolving Paradigm. Nat. Rev. Cancer 2013, 13, 714–726.
- Su, N.; Wang, P.; Li, Y. Role of Wnt/β-Catenin Pathway in Inducing Autophagy and Apoptosis in Multiple Myeloma Cells. Oncol. Lett. 2016, 12, 4623–4629.
- Chen, M.; Wang, Y.; Su, H.; Mao, L.; Jiang, X.; Zhang, T.; Dai, X. Three-Dimensional Electrochemical DNA Biosensor Based on 3D Graphene-Ag Nanoparticles for Sensitive Detection of CYFRA21-1 in Non-Small Cell Lung Cancer. Sensors Actuators B Chem. 2018, 255, 2910–2918.
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Molecular Mechanisms of Ferroptosis and Its Role in Cancer Therapy. J. Cell. Mol. Med. 2019, 23, 4900–4912.
- Nie, Q.; Hu, Y.; Yu, X.; Li, X.; Fang, X. Induction and Application of Ferroptosis in Cancer Therapy. Cancer Cell Int. 2022, 22, 12.
- Song, X.; Long, D. Nrf2 and Ferroptosis: A New Research Direction for Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 267.
- Feng, L.; Zhao, K.; Sun, L.; Yin, X.; Zhang, J.; Liu, C.; Li, B. SLC7A11 Regulated by NRF2 Modulates Esophageal Squamous Cell Carcinoma Radiosensitivity by Inhibiting Ferroptosis. J. Transl. Med. 2021, 19, 367.
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.Y.; Lin, L.T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad Targeting of Resistance to Apoptosis in Cancer. Semin. Cancer Biol. 2015, 35, S78–S103.
- Li, Y.; Yan, H.; Xu, X.; Liu, H.; Wu, C.; Zhao, L. Erastin/Sorafenib Induces Cisplatin-Resistant Non-Small Cell Lung Cancer Cell Ferroptosis through Inhibition of the Nrf2/XCT Pathway. Oncol. Lett. 2020, 19, 323.
- BioRender. Available online: https://biorender.com/ (accessed on 25 January 2022).
- Tao, N.; Li, K.; Liu, J. Molecular Mechanisms of Ferroptosis and Its Role in Pulmonary Disease. Oxid. Med. Cell. Longev. 2020, 2020, 9547127.
- Hao, S.; Liang, B.; Huang, Q.; Dong, S.; Wu, Z.; He, W.; Shi, M. Metabolic Networks in Ferroptosis (Review). Oncol. Lett. 2018, 15, 5405–5411.
- Walters, R.; Mousa, S.A. Modulations of Ferroptosis in Lung Cancer Therapy. Expert Opin. Ther. Targets 2022, 26, 133–143.
- Chlanda, A.; Walejewska, E.; Kowiorski, K.; Heljak, M.; Swieszkowski, W.; Lipińska, L. Investigation into Morphological and Electromechanical Surface Properties of Reduced-Graphene-Oxide-Loaded Composite Fibers for Bone Tissue Engineering Applications: A Comprehensive Nanoscale Study Using Atomic Force Microscopy Approach. Micron 2021, 146, 103072.
- Chlanda, A.; Kowiorski, K.; Małek, M.; Kijeńska-Gawrońska, E.; Bil, M.; Djas, M.; Strachowski, T.; Swieszkowski, W.; Lipińska, L. Morphology and Chemical Purity of Water Suspension of Graphene Oxide FLAKES Aged for 14 Months in Ambient Conditions. A Preliminary Study. Materials 2021, 14, 4108.
- Wu, T.; Liang, X.; Liu, X.; Li, Y.; Wang, Y.; Kong, L.; Tang, M. Induction of Ferroptosis in Response to Graphene Quantum Dots through Mitochondrial Oxidative Stress in Microglia. Part. Fibre Toxicol. 2020, 17, 30.
- Wu, T.; Wang, X.; Cheng, J.; Liang, X.; Li, Y.; Chen, M.; Kong, L.; Tang, M. Nitrogen-Doped Graphene Quantum Dots Induce Ferroptosis through Disrupting Calcium Homeostasis in Microglia. Part. Fibre Toxicol. 2022, 19, 22.
- Chen, Y.; Rivers-Auty, J.; Cricǎ, L.E.; Barr, K.; Rosano, V.; Arranz, A.E.; Loret, T.; Spiller, D.; Bussy, C.; Kostarelos, K.; et al. Dynamic Interactions and Intracellular Fate of Label-Free, Thin Graphene Oxide Sheets within Mammalian Cells: Role of Lateral Sheet Size. Nanoscale Adv. 2021, 3, 4166–4185.
- Pandit, S.; Gaska, K.; Kádár, R.; Mijakovic, I. Graphene-Based Antimicrobial Biomedical Surfaces. ChemPhysChem 2021, 22, 250–263.
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Ivanova, E.P. Mechano-Bactericidal Mechanism of Graphene Nanomaterials. Interface Focus 2018, 8, 20170060.
- Pierini, F.; Lanzi, M.; Nakielski, P.; Pawłowska, S.; Zembrzycki, K.; Kowalewski, T.A. Electrospun Poly(3-Hexylthiophene)/Poly(Ethylene Oxide)/Graphene Oxide Composite Nanofibers: Effects of Graphene Oxide Reduction. Polym. Adv. Technol. 2016, 27, 1465–1475.
- Shin, Y.C.; Lee, J.H.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Kim, B.; Park, J.C.; Han, D.W. Synergistic Effects of Reduced Graphene Oxide and Hydroxyapatite on Osteogenic Differentiation of MC3T3-E1 Preosteoblasts. Carbon 2015, 95, 1051–1060.
- Bebber, C.M.; Müller, F.; Clemente, L.P.; Weber, J.; von Karstedt, S. Ferroptosis in Cancer Cell Biology. Cancers 2020, 12, 164.
- Huang, G.; Chen, H.; Dong, Y.; Luo, X.; Yu, H.; Moore, Z.; Bey, E.A.; Boothman, D.A.; Gao, J. Superparamagnetic Iron Oxide Nanoparticles: Amplifying Ros Stress to Improve Anticancer Drug Efficacy. Theranostics 2013, 3, 116–126.
- Yu, X.; Zhang, Y.-C.; Yang, X.; Huang, Z.; Zhang, T.; Yang, L.; Meng, W.; Liu, X.; Gong, P.; Forni, A.; et al. Bonsai-Inspired AIE Nanohybrid Photosensitizer Based on Vermiculite Nanosheets for Ferroptosis-Assisted Oxygen Self-Sufficient Photodynamic Cancer Therapy. Nano Today 2022, 44, 101477.
- Pan, W.L.; Tan, Y.; Meng, W.; Huang, N.H.; Zhao, Y.B.; Yu, Z.Q.; Huang, Z.; Zhang, W.H.; Sun, B.; Chen, J.X. Microenvironment-Driven Sequential Ferroptosis, Photodynamic Therapy, and Chemotherapy for Targeted Breast Cancer Therapy by a Cancer-Cell-Membrane-Coated Nanoscale Metal-Organic Framework. Biomaterials 2022, 283, 121449.
- Li, Q.; Su, R.; Bao, X.; Cao, K.; Du, Y.; Wang, N.; Wang, J.; Xing, F.; Yan, F.; Huang, K.; et al. Glycyrrhetinic Acid Nanoparticles Combined with Ferrotherapy for Improved Cancer Immunotherapy. Acta Biomater. 2022, 144, 109–120.
- Zheng, Y.; Li, X.; Dong, C.; Ding, L.; Huang, H.; Zhang, T.; Chen, Y.; Wu, R. Ultrasound-Augmented Nanocatalytic Ferroptosis Reverses Chemotherapeutic Resistance and Induces Synergistic Tumor Nanotherapy. Adv. Funct. Mater. 2022, 32, 2107529.
- Pan, X.; Qi, Y.; Du, Z.; He, J.; Yao, S.; Lu, W.; Ding, K.; Zhou, M. Zinc Oxide Nanosphere for Hydrogen Sulfide Scavenging and Ferroptosis of Colorectal Cancer. J. Nanobiotechnol. 2021, 19, 392.
- Xu, R.; Yang, J.; Qian, Y.; Deng, H.; Wang, Z.; Ma, S.; Wei, Y.; Yang, N.; Shen, Q. Ferroptosis/Pyroptosis Dual-Inductive Combinational Anti-Cancer Therapy Achieved by Transferrin Decorated NanoMOF. Nanoscale Horizons 2021, 6, 348–356.
- Hsieh, C.-H.; Hsieh, H.-C.; Shih, F.-S.; Wang, P.-W.; Yang, L.-X.; Shieh, D.-B.; Wang, Y.-C. An Innovative NRF2 Nano-Modulator Induces Lung Cancer Ferroptosis and Elicits an Immunostimulatory Tumor Microenvironment. Theranostics 2021, 11, 7072.
- Li, Z.; Wu, X.; Wang, W.; Gai, C.; Zhang, W.; Li, W.; Ding, D. Fe(II) and Tannic Acid-Cloaked MOF as Carrier of Artemisinin for Supply of Ferrous Ions to Enhance Treatment of Triple-Negative Breast Cancer. Nanoscale Res. Lett. 2021, 16, 37.
- Tang, H.; Li, C.; Zhang, Y.; Zheng, H.; Cheng, Y.; Zhu, J.; Chen, X.; Zhu, Z.; Piao, J.G.; Li, F. Targeted Manganese Doped Silica Nano GSH-Cleaner for Treatment of Liver Cancer by Destroying the Intracellular Redox Homeostasis. Theranostics 2020, 10, 9865–9887.
- Sang, M.; Luo, R.; Bai, Y.; Dou, J.; Zhang, Z.; Liu, F.; Xu, J.; Liu, W.; Feng, F. Mitochondrial Membrane Anchored Photosensitive Nano-Device for Lipid Hydroperoxides Burst and Inducing Ferroptosis to Surmount Therapy-Resistant Cancer. Theranostics 2019, 9, 6209.
