In recent years, drought and waterlogging stress have seriously affected the growth of plants due to extreme climate change; these stresses are an important limiting factor for global agricultural and forestry productivity [
1]. Over the past decade, the total area of the world’s drylands has increased dramatically, with a clear upward trend in the scope, extent and frequency of drought, resulting in a total global loss of crop production of approximately $30 billion [
2,
3]. Waterlogging is the second most important climate disaster after drought. Since the 1990s, the scope of waterlogging disasters has been expanding year by year, and the frequency has also been increasing [
4,
5]. Due to the frequency and severity of drought and waterlogging, the global vegetation loss caused by these stresses is equivalent. The response and adaptation mechanisms of plants have been the focus of physiological and ecological research related to water stress (including drought stress and waterlogging stress), and are also very important for breeding water-tolerant varieties.
2. Morphological Structure Responses to Water Stress in Plants
The response of plants to water stress is mainly reflected in leaves and roots, and their external morphological characteristics and internal anatomical structure can best reflect the adaptability to adverse environments [
16,
17,
18,
19] (
Table 1). Leaves are the most variable organs in long-term adaptation to the environment. They react similarly under drought and waterlogging stress, showing signs of etiolation, atrophy, curling, senescence and even abscission [
20,
21]. In some cases, stress resulted in stunted leaf growth and reduced leaf number and area [
22,
23,
24] (
Figure 1).
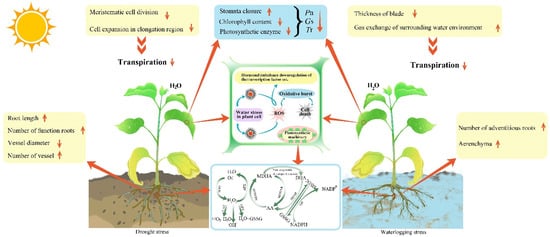
Figure 1. Changes to the morphological and anatomical structure of plant leaves and roots due to water stress. Pn: net photosynthetic rate; Gs: stomatal conductance; Tr: transpiration rate; ROS: reactive oxygen species; SOD: superoxide dismutase; CAT: catalase; APX: ascorbic peroxidase; GPX: peroxidase; GSSG: L-glutathione oxidized; MDHA: dehydroascorbic acid reductase; MDHAR: monodehydroascorbic acid reductase; DHAR: dehydroascorbate reductase glutathione; GR: glutathione reductase; GSH: glutathione peroxidase; AA: ascorbic acid.
2.1. Morphological Structure Responses to Drought Stress
Drought can limit plant growth by inhibiting the cell division of leaf meristematic tissue and cell expansion in elongation areas, as well as inducing complex changes in leaf thickness, palisade tissue and spongy tissue during adaptation [
25,
26,
27]. Rueda et al. [
28] found that the conifers (water-holding capacity of plants) could be improved by increasing the thickness of leaves and decreasing the thickness of palisade tissue and spongy tissue in drought environments. However, Zheng et al. [
29] found that
Lycium barbarum increased the thickness of palisade tissue and reduced the thickness of spongy tissue, inhibiting transpiration and preventing tissue from excessive dehydration. The above results presented that the internal structure of the leaf changes resulted in transpiration reduction, as well as photosynthetic rate.
The root is an important organ for plants to fix and absorb substances from the soil. Drought stress reduces the stele area, vessel diameter and secondary root cortex cells and increases the number of vessels in the stele to facilitate water flow [
30,
31,
32]. To improve water retention and drought resistance, plants not only extend the root system by increasing the number of functional roots, but also increase the water-absorbing capacity of the root sheath [
33,
34]. Furthermore, plants improve resistance by changing the root structure (such as root hair and root density) to influence root spatial distribution, soil fixation and nutrient absorption [
35,
36,
37]. Therefore, plants could improve water absorption capacity by changing root length and internal structure under drought stress conditions.
2.2. Morphological Structure Responses to Waterlogging Stress
The main response symptoms of leaves to waterlogging stress are curling, yellowing, wilting, falling off, rotting, etc. Leaves have two kinds of adaptation to waterlogging stress: one is to increase the thickness, while the other is to reduce the thickness. For the former, the water loss is reduced and the water holding capacity of plants is improved by increasing palisade tissue and spongy tissue, as well as the decrease in leaf and stomata size [
38,
39,
40]. The latter takes place because leaves cannot complete morphogenesis normally due to lack of water and nutrition [
41]. Thereby, some plants thin their leaves or form special leaves to promote the infiltration ability of CO
2 and inorganic nutrients into the leaves [
42,
43], and improve gas exchange to restore and maintain respiration under waterlogging stress [
44,
45]. Therefore, the internal anatomy variation of the leaf is to adjust the stomata and improve transpiration under waterlogging stress, but the reason is uncertain and further study is needed.
Aerenchyma forming in the adventitious roots are the most obvious adaptation features under waterlogging stress. Meanwhile, the epithelial cell wall keratinizes gradually under a waterlogged environment to promote oxygen capture by underwater tissue, and enhance waterlogging tolerance [
46,
47]. Yamauchi et al. [
48] found that there are a lot of root hairs in the adventitious roots, the surface area is large, and the cuticle of the adventitious root is thin, but the aerenchyma is well developed, which can improve the oxygen content of waterlogging-tolerant plants. Moreover, lignified and embolized vascular bundle cortical cells contribute to long-distance oxygen diffusion to the root tips, and block the entry of soil toxins into plants effectively. For instance, Ranathunge et al. [
49] found that rice promoted the early formation and increased lignin deposition in both the internal and external epidermis of roots, and prevented ion penetration more effectively under waterlogged conditions. Abiko et al. [
50] found that waterlogging-tolerant
teosinte formed adventitious roots and produced larger aerenchyma, a stronger lignified vascular bundle cell barrier, and the transport of oxygen from stem base to root tip was better than normal maize under a waterlogging environment. Therefore, the ways of producing adventitious roots are diverse in different types of plants under waterlogging stress, and strong waterlogging-tolerant plants are more likely to have the ability to form adventitious roots. It has been indicated that roots could improve adaptability by creating air cavities in the aerenchyma to expand storage space, and block the entry of soil toxins into plants.
Table 1. Characteristics of plant roots and leaves under water stress.
|
Treatment
|
Root
|
Reference
|
Leaf
|
Reference
|
|
Drought stress
|
Root system lengthens;
functional root number increases;
distribution breadth increases.
|
[2,51]
|
Wilting; crimping;
stomatal closure.
|
[52,53]
|
|
Area of the stele reduces;
number of vascular bundles increases but their diameter reduces.
|
[2,54,55]
|
Thickness of spongy tissue decreases; vascular bundles increase.
|
[56,57]
|
|
Waterlogging stress
|
Number of roots decreases;
root activity decreases;
adventitious roots are generated.
|
[58,59,60,61]
|
Etiolation; wilting; abscission;
stomatal closure.
|
[62,63,64]
|
|
Aerenchyma is formed in adventitious roots;
size of the stele reduces.
|
[65,66,67]
|
Blade thickness is reduced;
number and area of leaves decreases.
|
[62,68,69]
|
3. Photosynthetic Characteristics of Plant Responses to Water Stress
3.1. Photosynthetic Characteristics of Plant Responses to Drought Stress
To maintain photosynthesis, plants form a series of defense mechanisms to protect their photosynthetic organs from damage in the process of adapting to water stress [
70,
71]. For most plants, light water stress can control stomata and transpiration, directly regulate leaf water potential, and self-repair after a return to a normal water supply; some plants even increase photosynthesis [
72,
73]. For example, light drought stress usually leads to a stomatal conductance and transpiration increase, while moderate and severe drought stress results in a net photosynthetic rate (
Pn), stomatal conductance (
Gs) and transpiration rate (
Tr) decrease. However, the intercellular carbon dioxide concentration (
Ci) shows a different trend.
Ci increases or decreases with the deepening of stress, while the stomatal limit (
Ls) first increases and then decreases. These results indicate that the decrease in
Pn under drought stress is mainly caused by nonstomatal factors [
74,
75]. Most nonstomatal factors, including chlorophyll content, photosynthetic enzyme activity and active oxygen metabolism, are induced by moderate and severe drought stress. Drought not only inhibits the formation of chlorophyll directly [
76,
77], but also causes difficulty in absorbing mineral elements from the soil, causing leaf nutrient deficiency (for example, leaf etiolation) [
78,
79] (
Figure 1). The regulation of photosynthetic enzymes is a very complicated process. Light drought stress may slightly affect the photosynthetic carboxylation efficiency, but it can inhibit the activity of RuBPCase, which may result in a decrease in the photosynthetic carboxylation efficiency under severe drought stress [
80].
3.2. Photosynthetic Characteristics of Plant Responses to Waterlogging Stress
Under waterlogging stress, both stomatal and nonstomatal factors inhibit photosynthesis. For stomatal factors, the chemical signals from roots are transferred to the ground, forcing the stomata of leaves to close, and reducing the photosynthetic rate by decreasing the absorption capacity of the photosynthetic substrate CO
2 [
81,
82,
83]; Another aspect of stomatal conductance increasing is the supply of CO
2, which increases the amount of assimilates to maintain growth under waterlogging. For non-stomatal factors, there is the anaerobic respiration of the plant under hypoxic surroundings. Lactic acid and ethanol are produced, which break the balance of active oxygen metabolism, degrade chlorophyll and damage the photosynthetic apparatus, producing excess excitation energy and causing photoinhibition [
84,
85]. For severe waterlogging-tolerant plants, the stomata closed quickly due to the stress reaction of plants at the initial stage. For poor waterlogging-tolerant plants, leaf carbohydrates may accumulate rapidly within a few days, because root anaerobic respiration restrains sugar transfer from the stem to the root by reducing sugar consumption in the root, and the accumulation of photoassimilated products in leaves can form a negative feedback inhibition to the photosynthetic rate.
4. Antioxidant System of Plant Responses to Water Stress
Under normal physiological activities, plants produce reactive oxygen species (ROS), such as superoxide anion radicals (O
2−), singlet oxygen (O
2), hydroxyl radicals (·OH) and hydrogen peroxide (H
2O
2), as signal transmitters to regulate gene and protein expression in plant cells, and the production and elimination of ROS are always in a state of dynamic equilibrium [
86]. When the plant is stressed, the balance will be broken, the physiological and biochemical functions of the plant cell membrane will be disturbed, and the production of reactive oxygen species will increase [
87]. Plants have similar responses to drought and waterlogging, and both stresses activate the antioxidant defense system of plants to avoid cell damage. The components of the antioxidant defense system are enzymatic and nonenzymatic antioxidants. The enzymatic antioxidants include superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), glutathione reductase (GR), dehydroascorbate reductase glutathione (DHAR) and monodehydroascorbic acid reductase (MDHAR). The nonenzymatic antioxidants are glutathione (GSH), ascorbic acid (AA) (both water soluble), carotenoids and tocopherols (lipid soluble). Both components counteract the harm caused by reactive oxygen species [
88,
89,
90,
91].
The response of antioxidant enzymes in plants to water stress is mainly related to tolerance and the level of stress. The activity of SOD in leaves and roots of the same species increases with an increasing level of water stress. Furthermore, the disproportionation conversion of O
2− to H
2O
2 increases and the content of O
2− decreases. POD and CAT decompose H
2O
2 to H
2O, inhibit the accumulation of H
2O
2 effectively, protect plants from oxidative damage, and reduce the toxic effect on plants caused by water stress [
92]. This mechanism has been demonstrated in mosses [
93], trifoliate orange seedlings [
94], and tobacco [
95]. There are different antioxidant enzyme activities in different tolerant varieties under the same water stress. The adaptive mechanism of plants is a very complicated process, and there are no fixed rules to follow. For example, the SOD activity of
Poa pratensis and
Festuca arundinacea increased briefly and then decreased, while the CAT activity of
F. arundinacea decreased with increasing drought stress [
96]. The SOD activity of the drought-sensitive cultivar
Trifolium repens was inhibited under stress, but there was no significant change in the drought-tolerant cultivar Debut, which may be related to its higher ability to mitigate oxidative damage [
97]. These results showed that plants could increase the activity of antioxidant enzymes to cope with adverse environments, but the dynamic changes across individuals and stress degrees.