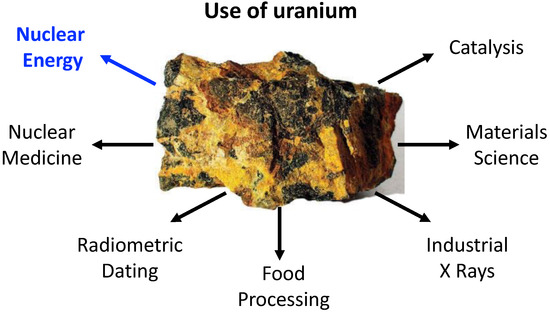
Uranium was discovered in 1789 by the German chemist Martin Heinrich Klaproth. It is the most known and used actinide element mainly because of its usage in nuclear fuel processing; however, the application potential of uranium compounds is much broader, stretching, e.g., into the field of organometallic synthesis, catalysis and beyond. Moreover, uranium is one of the few naturally occurring actinides, whereas the other members, with the exception of thorium, are considered to be human-made, despite their natural occurrence in traces as a result of uranium and thorium spontaneous fissions. In nature, uranium occurs as three main isotopes, where the non-fissile 238U is the most abundant, encompassing over 99% of the available uranium resources.
1. Introduction
Uranium was discovered in 1789 by the German chemist Martin Heinrich Klaproth [
1]. It is the most known and used actinide element mainly because of its usage in nuclear fuel processing; however, the application potential of uranium compounds is much broader, stretching, e.g., into the field of organometallic synthesis, catalysis and beyond, as sketched in
Figure 1 [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13]. Moreover, uranium is one of the few naturally occurring actinides [
1], whereas the other members, with the exception of thorium, are considered to be human-made, despite their natural occurrence in traces as a result of uranium and thorium spontaneous fissions. In nature, uranium occurs as three main isotopes, where the non-fissile
238U is the most abundant, encompassing over 99% of the available uranium resources.
Figure 1. Examples of the broad range of uranium use.
In the Earth’s crust, there are different types of uranium ores, such as uraninite or pitchblende (
xUO
2⋅yUO
3, with
y/
x < 2), carnotite (K
2O·2UO
3⋅V
2O
5⋅xH
2O) and autunite (CaO·2UO
3⋅P
2O
5⋅xH
2O) [
14,
15]. In order to extract uranium from its ore, chemical processes utilizing acids and oxidizing agents are employed. Often, specific isotopic separation processes have to be applied in order to acquire the desired concentration of a given uranium isotope for energy generation purposes [
1]. The most used isotopic separation process involves uranium conversion into the gaseous UF
6 followed by isotope separation due to mass differences [
16].
A number of reviews have been previously published covering the industrial use of uranium, i.e., in energy supply and related fields [
15,
17,
18,
19,
20,
21]. Organouranium compounds, i.e., the uranium equivalent of organometallic complexes, have attracted considerable attention, including reviews on (
η8-C
8H
8)
2U and actinides cyclobutadienyl complexes, respectively [
22,
23].
Uranium coordination chemistry, in general, has been being widely investigated [
9,
24,
25,
26], especially with regard to possible applications in nuclear waste treatment [
27], where the efficient extraction of uranium from the waste generated during the nuclear fuel cycle (so-called spent nuclear fuel), and its recycling into the nuclear fuel process is an important step to reduce long-term radioactive waste [
28,
29,
30,
31,
32]. Uranium oxides have been used for a long time as nuclear fuel [
33,
34]. However, their handling in the spent nuclear fuel regeneration process is difficult [
35].
2. Role of Uranium in the Nuclear Fuel Cycle
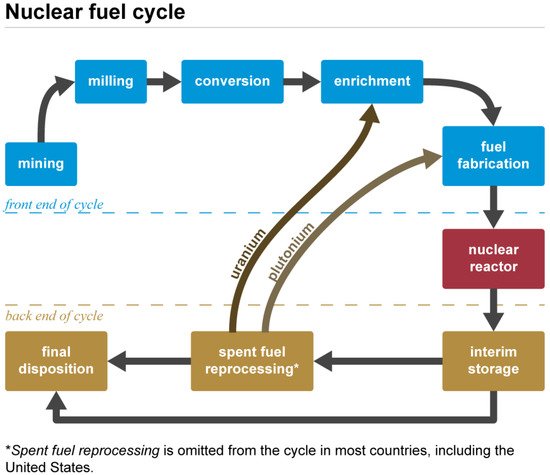
Uranium is mainly used in the nuclear fuel cycle sketched in
Figure 2, an industrial process involving various activities to produce electricity from uranium in nuclear power reactors, i.e., the progression of nuclear fuel from creation to disposal. Typically, uranium is employed in the form of UO
2. During uranium decay, fissile plutonium is produced. As spent fuel can be reprocessed and recycled, this explains the presence of a plutonium admixture with uranium in the subsequent fuel cycle [
14,
93].
Figure 2. Nuclear fuel cycle. Source: Pennsylvania State University Radiation Science and Engineering Center (public domain).
Uranium is a relatively common element that is found throughout the world and mined in a number of countries. After mining, uranium ore is crushed in a mill and grounded to a fine slurry, which is leached in sulfuric acid (or sometimes a strong alkaline solution) to allow the separation of uranium from the waste rock. It is then recovered from solution and precipitated as uranium oxide concentrate, mostly U
3O
8, which is sometimes referred to as
yellowcake uranium (caused by its khaki color). U
3O
8 concentrate typically contains more than 80% uranium. The original ore, by comparison, may contain as little as 0.1% uranium [
14,
93].
Often, the yellowcake needs to be highly purified, due to impurities originated from the ore that remain, a process that is highly specific and depends on the ore quality [
94]. Alternate processes have been proposed, such as supercritical carbon dioxide extraction, in particular for phosphate ores [
95,
96]. Purified U
3O
8 is the product that is sold. About 200 tonnes are required to keep a large (1000 MWe) nuclear power reactor generating electricity for a year.
The uranium oxide obtained after uranium milling is not directly usable as fuel for a nuclear reactor—additional processing is required. Only 0.7% of natural uranium is fissile—capable of undergoing fission, the process by which energy is produced in a nuclear reactor. The form (or isotope) of uranium that is fissile is
235U, whereas the remainder is
238U. About 3.5–5% of the fissile isotope
235U is required to maintain the chain reaction. Therefore, a physical process commonly called
uranium enrichment has to be invoked to increase (or ‘enrich’) the
235U concentration, which requires uranium to be in a gaseous form [
1]. U
3O
8 is first refined to uranium dioxide UO
2, which can be directly used as fuel for those types of reactors that do not require enriched uranium. For reactors requiring enriched uranium, UO
2 is converted into uranium hexafluoride, which is a gas at relatively low temperatures. In order to produce UF
6, UO
2 is first converted into the solid UF
4, followed by oxidation with F
2, yielding UF
6(g).
This route is often preferable instead of the direct conversion of the oxide into the fluoride, due to the lower fluorine demand. Solid state reactions often lead to byproducts, which also have to be taken into account [
94,
97,
98,
99]. A gas-phase mechanism for such reactions was previously studied theoretically [
100]. Currently, centrifuges are used for U-enrichment processes. Nonetheless, there are alternative techniques, such as gaseous diffusion, which was widely used in the past, and laser-based processes, which are still in development [
101].
Subsequently, uranium has to be re-converted to its oxide form. There are several processes available, including wet conversions employing aqueous ammonia, for example, which hydrolyses UF
6, followed by calcination. An alternative process is dry conversion, which is known to be less injurious for the environment. Independent of the method used, it is important that the final product—the actual reactor fuel—has the correct oxygen to uranium ratio [
93]. In order to increase the stability and strength, nuclear fuel can be alloyed with zirconium [
14,
93,
102], which has also drawn attention due to its potential use in spent fuel reprocessing [
103,
104].
The fission process transforms U (and Pu) into lighter elements, denoted as
fissile products, which are often also radioactive. Thus, there are safety concerns regarding nuclear waste. Spent fuel still contains large amounts of uranium, known as depleted uranium and, therefore, can be recycled and reused from nuclear waste [
14]; however, the often highly radioactive fissile products have to be safely stored [
14,
105].
As alternative to uranium oxides, uranium nitrides are discussed as novel nuclear fuel as they display higher values for thermal conductivity and melting point [
106]. The species N≡U≡N was first obtained in an argon matrix through a molecular nitrogen reaction with atomic uranium. Clearly, uranium has the potential to break the triple bond in N
2 [
107]. A recent investigation of N≡U≡N in both neutral and negatively charged states has led to new insights into the properties of this molecule in both the ground and excited states [
108].
Other uranium nitrides followed, such as the related NUO, that was first observed in solid argon as a product of the reaction between U and NO, and density functional theory calculations predicted a duplet spin state [
109]. NUO was also synthesized in solid neon by Zhou and Andrews [
110]. A related species, NUNH, was observed as a product of the N≡U≡N reaction with hydrogen atoms, suggesting that NUNH features both double and triple uranium-nitrogen bonds [
111].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23094655