To facilitate the glycerol purification, it is important to know the composition of glycerol from the biodiesel obtained from waste oils and how these oils and the type of biodiesel synthesis influence the glycerol composition. First, different glycerol impurities were studied based on the oil origin and the used catalysts. Thus, the glycerol impurities are detailed to show their influence on the glycerol or on its transformation in other products. Different glycerol characterization techniques were used to determine the purity level and properties of glycerol. The main interest of this part is to describe different methods to purify glycerol and to understand its composition and its utilization.
- biodiesel
- bioprocesses
- waste oils
- crude glycerol
- purification
- valorization
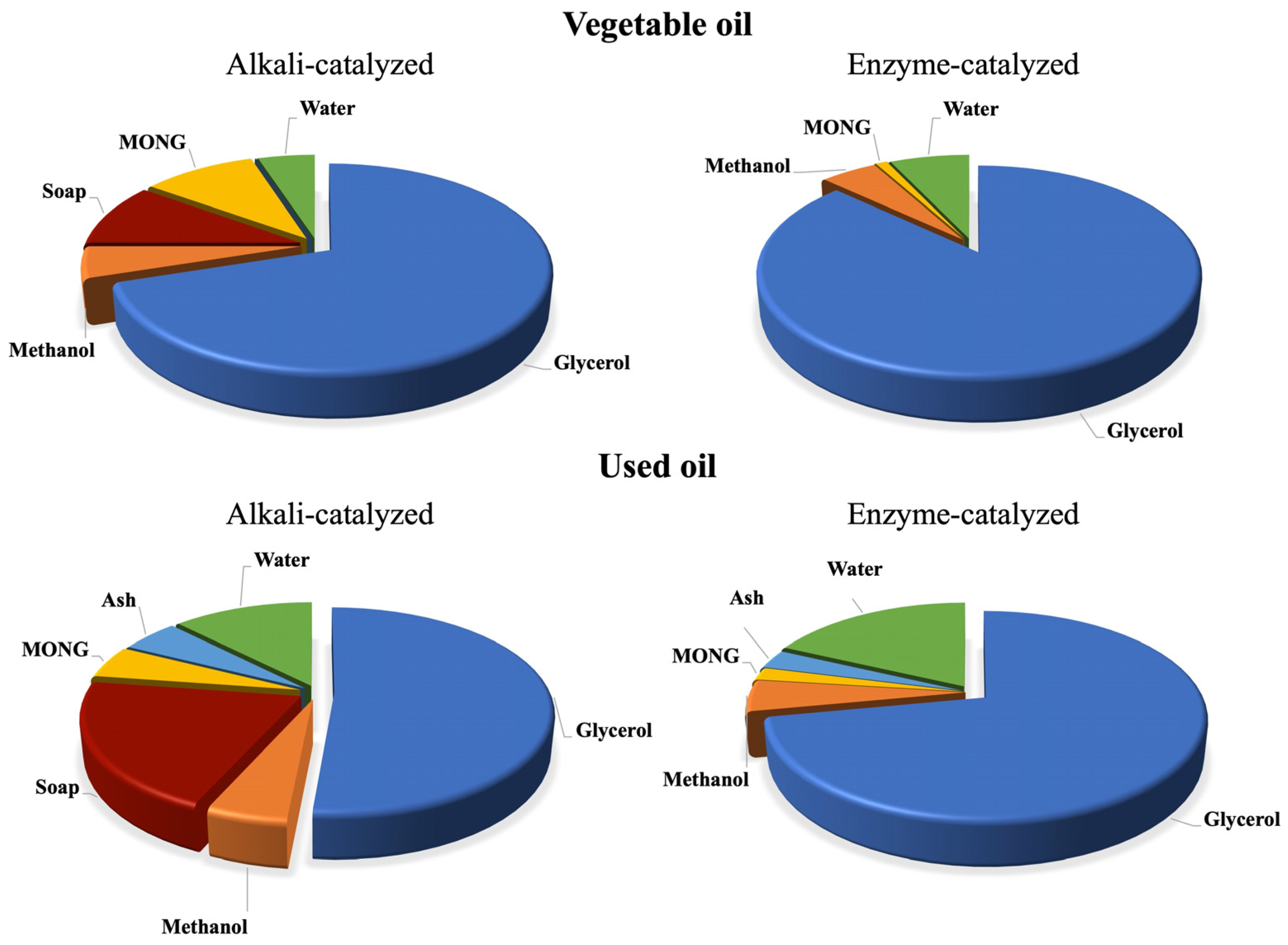
1. Purity of Glycerol and Influence of Its Impurities
2. Glycerol Characterization Techniques
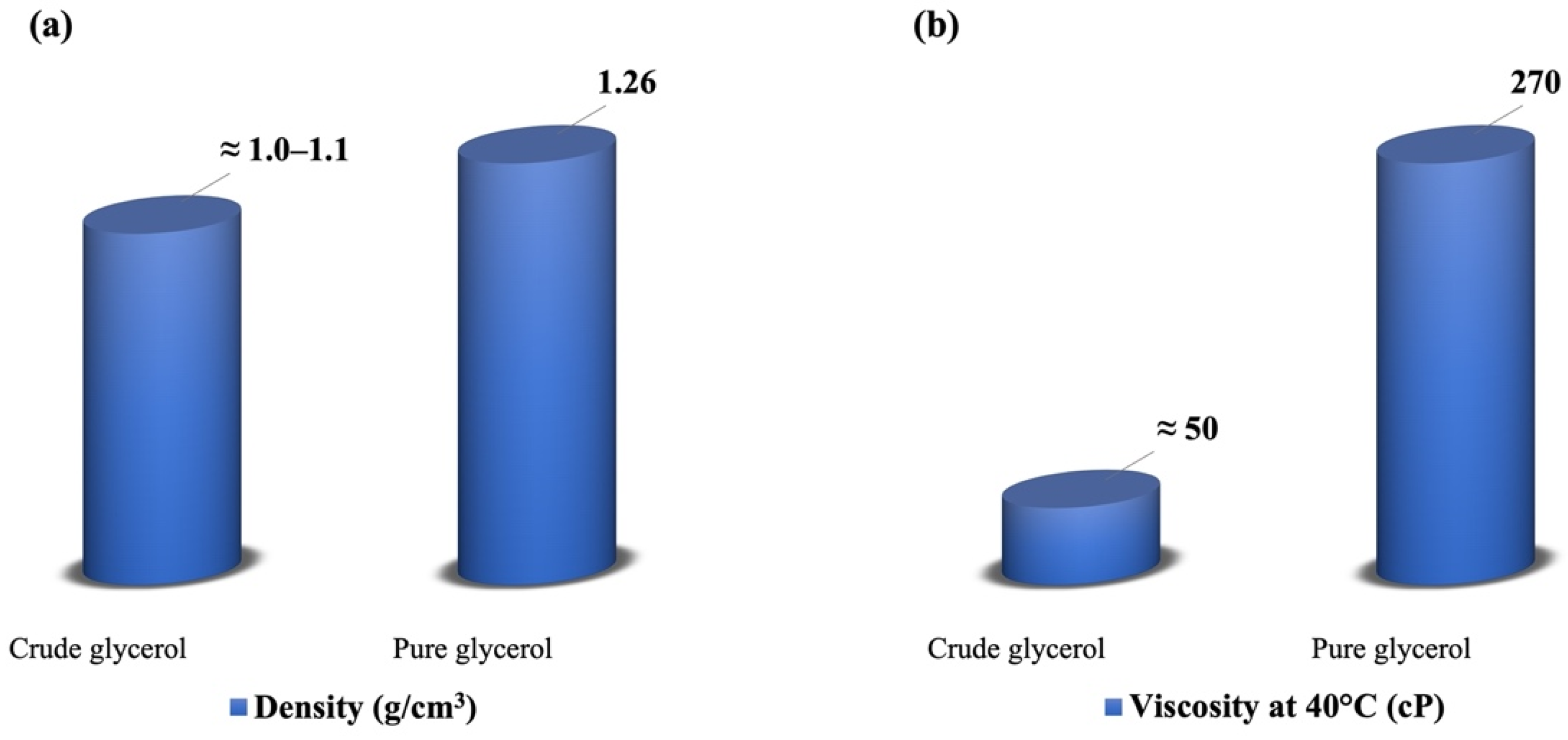
2.1. Properties of Glycerol
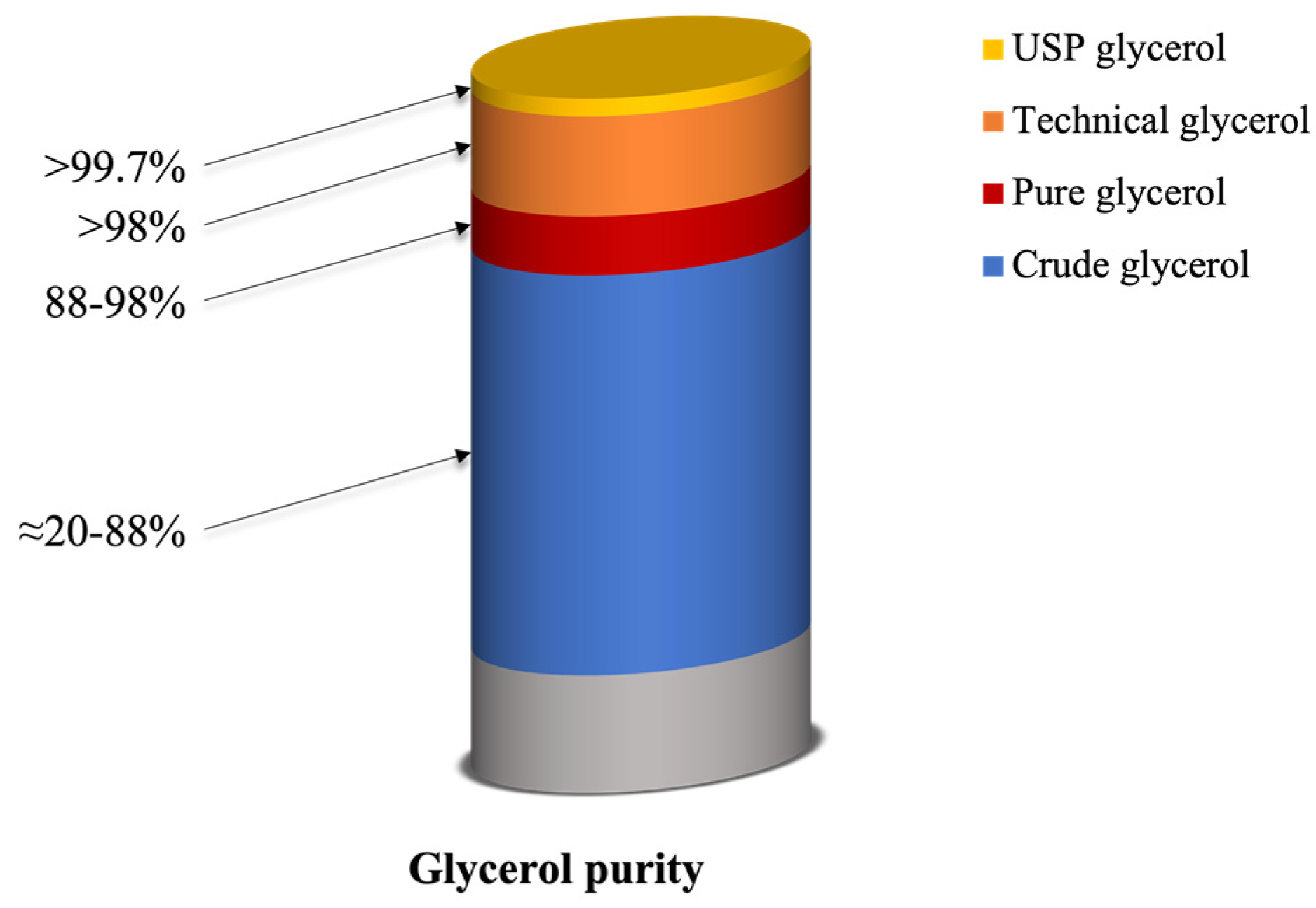
2.2. Purity of Glycerol
2.3. Impurities Measurement
3. Glycerol Purification Methods
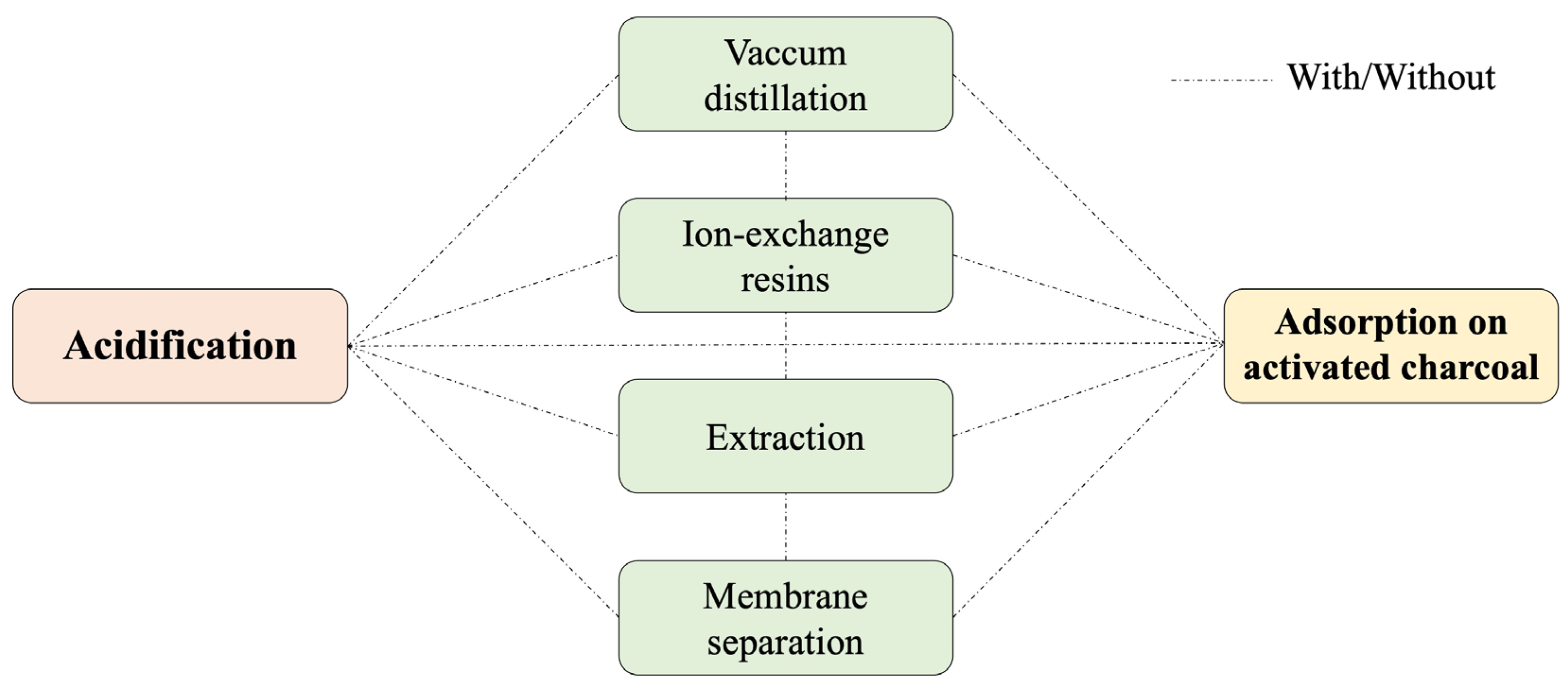
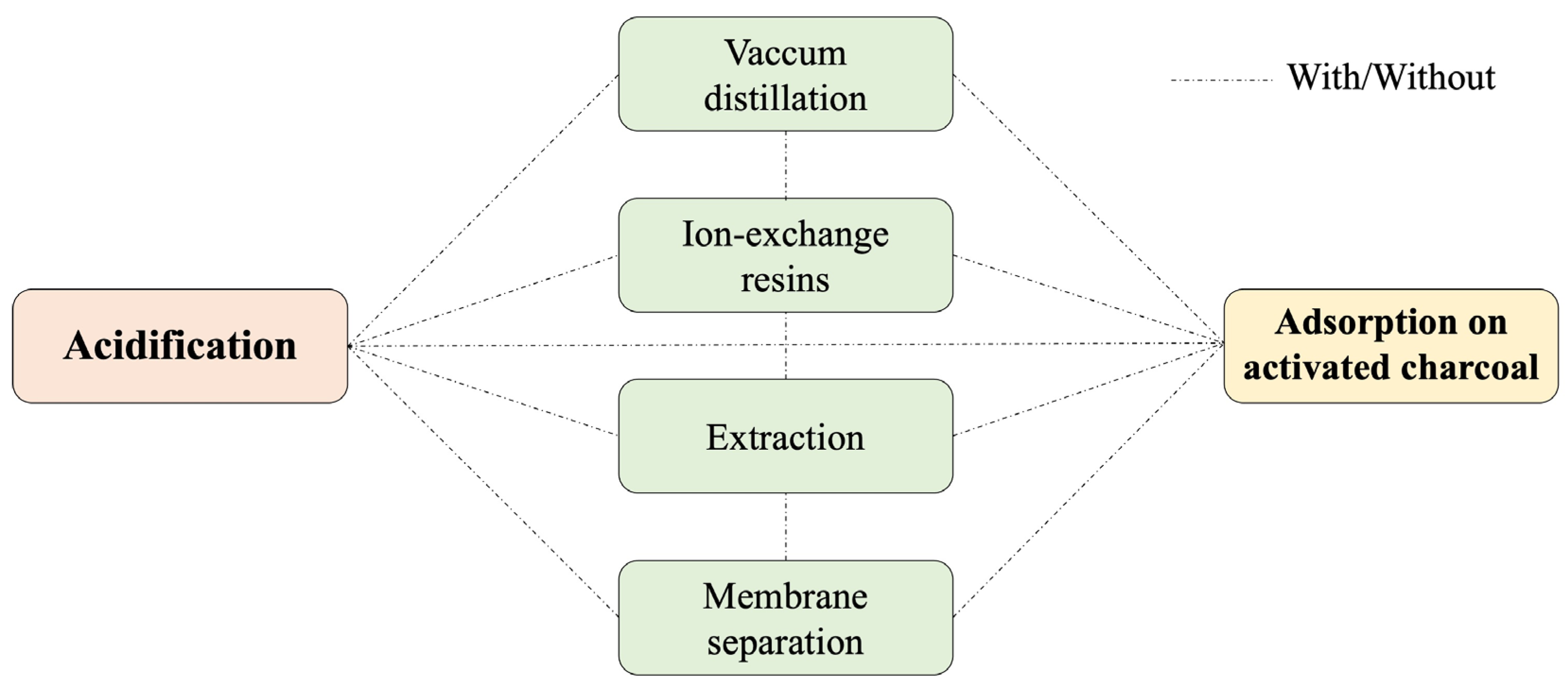
3.1. Acidification/Neutralization
3.2. Vacuum Distillation
3.3. Ion-Exchange Resins
3.4. Adsorption on Activated Charcoal
3.5. Extraction
3.6. Membrane Separation
3.7. Multi-Step Methods
| Type of Oil | Purification Techniques | Glycerol (%) | Purified Glycerol (%) | Ref. |
|---|---|---|---|---|
| Waste oil |
|
36.7 | 96.2 | [53] |
| Waste oil |
|
29.8 | 99.0 | [54] |
| Waste oil (lab. scale) |
|
51.88 | 78.72 | [55] |
| Waste oil (ind. scale) |
|
29.99 | 60.6 | [55] |
| Waste oil |
|
40.6 | 96.08 | [56] |
| N.I. |
|
12.0 | 96 | [57] |
| Waste oil |
|
74.0 | 99.2 | [58] |
| Waste oil |
|
35.66 | 97.37 | [59] |
| N.I. |
|
40.0 | 97.5 | [60] |
| Virgin (hemp) oil |
|
51.38 | 93.89 | [61] |
| N.I. |
|
40.00 | 93.70 | [62] |
| Virgin (canola) oil |
|
N.I. | 98.1 | [63] |
| N.I. |
|
N.I. | N.I. | [64] |
4. Possible Applications of Glycerol Purification Methods from Biodiesel Produced by Enzymatic Processes on Waste Oils
5. Bioconversion of Glycerol from Waste Oils to High Value-Added Products
| Glycerol (%) | Glycerol Treatment | Final Product | Process or Microorganism | Temperature (°C) | Time (h) | Yield | Ref. |
|---|---|---|---|---|---|---|---|
| N.I. |
|
Methane | Anerobic digestion | 25 | 2 | 54% | [68] |
| N.I. |
|
Methane | Anerobic digestion | 2 | 0.8 | 0.306 m3 CH4/kg | [69] |
| 83.41 |
|
Glycolipids | Ustilago maydis | 30 | 196.8 | 32.1 g/L | [70] |
| N.I. |
|
Ethanol | Klebsiella variicola | 25 | 24 | 9.8 g/L | [71] |
| 47.5 |
|
Hydrogen | E. coli/Enterobacter spH1 | 37 | 120 | 69.1 mM | [72] |
| 10.41 |
|
Hydrogen | Anerobic digestion | 37 | 19.1 | 2.2 mol H2 L−1 | [73][74] |
| N.I. |
|
Lactic acid | Rhizopus microsporus | 37 | 1.3 | 1.33 g/L | [75] |
| 49.30 |
|
1,3-propanediol / lactate | E. coli/Enterobacter spH1 | 37 | 1.3 | 27.77 g/L 1,3-PDO 14.68 g/L LA |
[76] |
| 69 |
|
1,3-propanediol | Klebsiella pneumoniae | 37 | 12 | 0.64 mol1,3-PDO/mol glycerol | [77] |
| 31.8 |
|
Biodiesel | Trichosporon oleaginosus | 28 | 72 | 5.24 g/L | [78] |
| 32.97 |
|
Triacylglycerols | Rhodosporidium toruloides | 30 | 160 | 13.4 g/L | [79] |
| 64.5 |
|
Biodiesel | Candida viwanathii Y-E4 | 30 | 166 | 13.6 g/L | [80] |
| 82 |
|
2-phenylethanol | Yarrowia lipolytica CH1/5 | 27 | 200 | 2.2 g/L | [81] |
| N.I. |
|
Serinol | Gluconobacter oxydans transaminase |
30 | 44 | 36 mM | [82] |
This entry is adapted from the peer-reviewed paper 10.3390/en15093381
References
- Robles-Medina, A.; González-Moreno, P.A.; Esteban-Cerdán, L.; Molina-Grima, E. Biocatalysis: Towards ever greener biodiesel production. Biotechnol. Adv. 2009, 27, 398–408.
- Gao, Z.; Ma, Y.; Wang, Q.; Zhang, M.; Wang, J.; Liu, Y. Effect of crude glycerol impurities on lipid preparation by Rhodosporidium toruloides yeast 32489. Bioresour. Technol. 2016, 218, 373–379.
- Sarma, S.J.; Dhillon, G.S.; Brar, S.K.; Bihan, Y.L.; Buelna, G.; Verna, M. Investigation of the effect of different crude glycerol components on hydrogen production by Enterobacter aerogenes NRRL B-407. Renew. Energy 2013, 60, 566–571.
- Yang, X.; Jin, G.; Gong, Z.; Shen, H.; Bai, F.; Zhao, Z.K. Recycling biodiesel-derived glycerol by the oleaginous yeast Rhodosporidium toruloides Y4 through the two-stage lipid production process. Biochem. Eng. J. 2014, 91, 86–91.
- Uprety, B.K.; Samavi, M.; Rakshit, S.K. Contribution of specific impurities in crude glycerol towards improved lipid production by Rhodosporidium toruloides ATCC 10788. Bioresour. Technol. Rep. 2018, 3, 27–34.
- Isahak, W.N.R.W.; Ramli, Z.A.C.; Ismail, M.; Jahim, J.M.; Yarmo, M.A. Recovery and purification of crude glycerol from vegetable oil transesterification. Sep. Purif. Rev. 2015, 44, 250–267.
- ASTM D5002-19; Standard Test Method for Density, Relative Density, and API Gravity of Crude Oils by Digital Density Analyzer. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D445-19a; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2019. Available online: www.astm.org (accessed on 16 June 2020).
- Harabi, M.; Bouguerra, S.N.; Marrakchi, F.; Chrysikou, L.P.; Bezergianni, S.; Bouaziz, M. Biodiesel and crude glycerol from waste frying oil: Production, characterization and evaluation of biodiesel oxidative stability with diesel blends. Sustainability 2019, 11, 1937.
- Hunsom, M.; Autthanit, C. Adsorptive purification of crude glycerol by sewage sludge-derived activated carbon prepared by chemical activation with H3PO4, K2CO3 and KOH. Chem. Eng. J. 2013, 229, 334–343.
- ASTM D1093-98; Standard Test Method for Acidity of Hydrocarbon Liquids and Their Distillation Residues. ASTM International: West Conshohocken, PA, USA, 1998.
- ASTM D240-19; Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D482-03; Standard Test Method for Ash from Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2003.
- ASTM D4377-00e1; Standard Test Method for Water in Crude Oils by Potentiometric Karl Fischer Titration. ASTM International: West Conshohocken, PA, USA, 2000.
- ASTM D4017-02(2015); Standard Test Method for Water in Paints and Paint Materials by Karl Fischer Method. ASTM International: West Conshohocken, PA, USA, 2015.
- Cocks, L.V.; Devine, J.; Gibbs, F.B. Determination of MONG in place of organic residue for glycerine analysis. Analyst 1970, 95, 278–283.
- Ardi, M.S.; Aroua, M.K.; Hashim, N.A. Progress, prospect and challenges in glycerol purification process: A review. Renew. Sustain. Energy Rev. 2015, 42, 1164–1173.
- Kumar, L.R.; Yellapu, S.K.; Tyagi, R.D.; Zhang, X. A review on variation in crude glycerol composition, bio-valorization of crude and purified glycerol as carbon source for lipid production. Bioresour. Technol. 2019, 293, 1221555.
- Hájek, M.; Skopal, F. Treatment of glycerol phase formed by biodiesel. Bioresour. Technol. 2010, 101, 3242–3245.
- Gil, S.; Marchena, M.; Fernández, C.M.; Sánchez-Silva, L.; Romero, A.; Valverde, J.L. Catalytic oxidation of crude glycerol using catalysts based on Au supported on carbonaceous materials. Appl. Catal. A Gen. 2013, 450, 189–203.
- Velázquez-Hernández, I.; Zamudio, E.; Rodríguez-Valadez, F.J.; García-Gómez, N.A.; Álvarez-Contreras, L.; Guerra-Balcázar, M.; Arjona, N. Electrochemical valorization of crude glycerol in alkaline medium energy conversion using Pd, Au and PdAu nanomaterials. Fuel 2020, 262, 116556.
- Posada, J.A.; Naranjo, J.M.; López, J.A.; Higuita, J.C.; Cardona, C.A. Design and analysis of poly-3-hydroxybutyrate processes from crude glycerol. Process Biochem. 2011, 46, 310–317.
- Skrzyńska, E.; Wondolowska-Grabowska, A.; Capron, M.; Dumeignil, F. Crude glycerol as a raw material for the liquid phase oxidation reaction. Appl. Catal. A Gen. 2014, 482, 245–257.
- Remón, J.; Jarauta-Córdoba, C.; García, L.; Arauzo, J. Analysis and optimization of H2 production from crude glycerol by steam reforming using a novel two step process. Fuel Process. Technol. 2016, 145, 130–147.
- Valerio, O.; Horvath, T.; Pond, C.; Misra, M.; Mohanty, A. Improved utilization of crude glycerol from biodiesel industries: Synthesis and characterization of sustainable biobased polyesters. Ind. Crops Prod. 2015, 78, 141–147.
- Luo, X.; Ge, X.; Cui, S.; Li, Y. Value-added processing of crude glycerol into chemicals and polymers. Bioresour. Technol. 2016, 215, 144–154.
- Nasir, N.F.; Mirus, M.F.; Ismail, M. Purification of crude glycerol from transesterification reaction of palm oil using direct method and multistep method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 243, 012015.
- Isahak, W.N.R.W.; Jahim, J.M.; Ismail, M.; Nasir, N.F.; Ba-Abbad, M.M.; Yarmo, M.A. Purification of crude glycerol from industrial waste: Experimental and simulation studies. J. Eng. Sci. Technol. 2016, 11, 1056–1072.
- Abdul Raman, A.A.; Tan, H.W.; Buthiyappan, A. Two-step purification of glycerol as a value added by product from the biodiesel production process. Front. Chem. 2019, 7, 774–782.
- Lopes, A.P.; Souza, P.R.; Bonafé, E.G.; Visentainer, J.V.; Martins, A.F.; Canesin, E.A. Purified glycerol is produced from the frying oil transesterification by combining a pre-purification strategy performed with condensed tannin polymer derivative followed by ionic exchange. Fuel Process. Technol. 2019, 187, 73–83.
- Rodrigues, A.; Bordado, J.C.; dos Santos, R.G. Upgrading the glycerol from biodiesel production as source of energy carriers and chemicals-A technological review for three chemical pathways. Energies 2017, 10, 1817.
- Tan, H.W.; Abdul Aziz, A.R.; Aroua, M.K. Glycerol production and its application as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127.
- Kongjao, S.; Damronglerd, S.; Hunsom, M. Purification of crude glycerol derived from waste used-oil methyl ester plant. Korean J. Chem. Eng. 2010, 27, 944–949.
- Demaman Oro, C.E.; Bonato, M.; Oliveira, J.V.; Tres, M.V.; Mignoni, M.L.; Dallago, R.M. A new approach for salts removal from crude glycerin coming from industrial biodiesel production unit. J. Environ. Chem. Eng. 2019, 7, 102883.
- Xiao, Y.; Xiao, G.; Varma, A. A universal procedure for crude glycerol purification from different feedstocks in biodiesel production: Experimental and simulation study. Ind. Eng. Res. 2013, 52, 14291–14296.
- Strathmann, H. Membrane separation processes: Current relevance and future opportunities. AIChE J. 2001, 47, 1077–1087.
- Gomes, M.C.S.; Pereirra, N.C.; de Barros, S.T.D. Separation of biodiesel and glycerol using ceramic membranes. J. Membr. Sci. 2010, 352, 271–276.
- Sdrula, N. A study using classical or membrane separation in the biodiesel process. Desalination 2010, 250, 1070–1072.
- Liang, B.; He, X.; Hou, J.; Li, L.; Tang, Z. Membrane separation in organic liquid: Technologies, achievements, and opportunities. Adv. Mater. 2018, 1806090.
- 2007 EET Corp., Web Information; HEEPMTM Technology: Harriman, TN, USA, 2007.
- Indok Nurul Hasyimah, M.A.; Mohammad, A.W.; Markom, M. Influence of triglycerides on fouling of glycerol-Water with ultrafiltration membranes. Ind. Eng. Chem. Res. 2011, 50, 7520–7526.
- Mah, S.K.; Leo, C.P.; Wu, T.Y.; Chai, S.P. A feasibility investigation on ultrafiltration of palm oil and oleic acid removal from glycerin solutions: Flux decline, fouling pattern, rejection and membrane characterizations. J. Membr. Sci. 2012, 389, 245–256.
- Jeromin, L.; Johannisbauer, W.; Blum, S.; Sedelies, R.; Moormann, H.; Holforth, B.; Plachenka, J. Process for the Purification of Crude Glycerol Water. U.S. Patent 5,527,974 A, 18 June 1996.
- Vadthya, P.; Kumari, A.; Sumana, C.; Sridhar, S. Electrodialysis aided desalination of crude glycerol in the production of biodiesel from oil feed stock. Desalination 2015, 362, 133–140.
- Kalafatakis, S.; Braekevelt, S.; Carlsen, V.; Lange, L.; Skiadas, I.V.; Gavala, H.N. On a novel strategy for water recovery and recirculation in biorefineries through application of forward osmosis membranes. Chem. Eng. J. 2017, 311, 209–216.
- Zulfikar, M.A.; Mohammad, A.W.; Hilal, N. Preparation and characterization of novel porous PMMA-SiO2 hybrid membranes. Desalination 2006, 192, 262–270.
- Xie, K.; Yu, Y.; Shi, Y. Synthesis and characterization of cellulose/silica hybrid materials with chemical crosslinking. Carbohydr. Polym. 2009, 78, 799–805.
- Shaari, N.Z.K.; Rahman, N.A. Performance of Thin Film Composite in the Purification of Crude Glycerol; IEEE Business Engineering and Industrial Applications Colloquium (BEIAC): Langkawi, Malaysia, 2013.
- Siyal, M.I.; Lee, C.K.; Park, C.; Khan, A.A.; Kim, J.O. A review of membrane development in membrane distillation for emulsified industrial or shale gas wastewater treatments with feed containing hybrid impurities. J. Environ. Manag. 2019, 243, 45–66.
- Shirazi, M.M.A.; Kargari, A.; Tabatabaei, M.; Ismail, A.F.; Matsuura, T. Concentration of glycerol wastewater using sweeping gas membrane distillation. Chem. Eng. Process. Process Intensif. 2014, 78, 58–66.
- Pal, P.; Chaurasia, S.P.; Upadhyaya, S.; Agarwal, M.; Sridhar, S. Glycerol purification using membrane technology. In Membrane Processes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 431–463.
- Yalcinkaya, F.; Boyraz, E.; Maryska, J.; Kucerova, K. A review on membrane technology and chemical surface modification for the oily wastewater treatment. Materials 2020, 13, 493.
- Manosak, R.; Limpattayanate, S.; Hunsom, M. Sequential-refining of crude glycerol derived from waste used-oil methyl ester plant via a combined process of chemical and adsorption. Fuel Process. Technol. 2011, 92, 92–99.
- Hunsom, M.; Saila, P.; Chaiyakam, P.; Kositnan, W. Comparison and combination of solvent extraction and adsorption for crude glycerol enrichment. Int. J. Renew. Energy Res. 2013, 3, 364–371.
- Pitt, F.D.; Domingos, A.M.; Chivanga Barros, A.A. Purification of residual glycerol recovered from biodiesel production. S. Afr. J. Chem. Eng. 2019, 29, 42–51.
- Javani, A.; Hasheminejad, M.; Tahvildari, K.; Tabatabaei, M. High quality potassium phosphate production through step-by-step glycerol purification: A strategy to economize biodiesel production. Bioresour. Technol. 2012, 104, 788–790.
- Nanda, M.R.; Yuan, Z.; Qin, W.; Poirier, M.A.; Chunbao, X. Purification of crude glycerol using acidification: Effects of acid types and product characterization. Austin J. Chem. Eng. 2014, 1, 7–13.
- Contreras-Andrade, I.; Avella-Moreno, E.; Sierra-Cantor, J.F.; Guerrero-Fajardo, C.A.; Sodré, J.R. Purification of glycerol from biodiesel production by sequential extraction monitored by 1H NMR. Fuel Process. Technol. 2015, 132, 99–104.
- Muniru, O.S.; Ezeanyanaso, C.S.; Fagbemigun, T.K.; Akubueze, E.U.; Oyewole, A.O.; Okunola, O.J.; Asieba, G.; Shifatu, A.O.; Igwe, C.C.; Elemo, G.N. Valorization of biodiesel production: Focus on crude glycerine refining/purification. J. Sci. Res. Rep. 2016, 11, 1–8.
- Dhabhai, R.; Ahmadifeijani, E.; Dalai, A.K.; Reaney, M. Purification of crude glycerol using a sequential physico-chemical treatment, membrane filtration, and activated charcoal adsorption. Sep. Purif. Technol. 2016, 168, 101–106.
- Sadhukhan, S.; Sarkar, U. Production of purified glycerol using sequential desalination and extraction of crude glycerol obtained during trans-esterification of Crotalaria juncea oil. Energy Convers. Manag. 2016, 118, 450–458.
- Chol, C.G.; Dhabhai, R.; Dalai, A.K.; Reaney, M. Purification of crude glycerol derived from biodiesel production process: Experimental studies and techno-economic analyses. Fuel Process. Technol. 2018, 178, 78–87.
- Sidhu, M.S.; Roy, M.M.; Wang, W. Glycerine emulsions of diesel-biodiesel blends and their performance and emissions in a diesel engine. Appl. Energy 2018, 230, 148–159.
- de Farias, B.S.; Vidal, É.M.; Ribeiro, N.T.; da Silveiro, N., Jr.; da Silva Vaz, B.; Kuntzler, S.G.; de Morais, M.G.; Cadaval, T.R.S., Jr.; de Almeida Pinto, L.A. Electrospun chitosan/poly(ethylene oxide) nanofibers applied for the removal of impurities from biodiesel production by biosorption. J. Mol. Liq. 2018, 268, 365–370.
- Hobden, R. Commercializing enzymatic biodiesel production. Biodiesel Mag. 2014, 11, 34–37.
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122.
- Vivek, N.; Sindhu, R.; Madhavan, A.; Anju, A.J.; Castro, E.; Faraco, V.; Pandey, A.; Binod, P. Recent advances in the production of value added chemicals and lipids utilizing biodiesel industry generated crude glycerol as a substrate–Metabolic aspects, challenges and possibilities: A overview. Bioresour. Technol. 2017, 239, 507–517.
- Nuchdang, S.; Phalakornkule, C. Anaerobic digestion of glycerol and co-digestion of glycerol and pig manure. J. Environ. Manag. 2012, 101, 164–172.
- Siles López, J.Á.; de los Ángeles Martín Santos, M.; Chica Pérez, A.F.; Martín, A.M. Anaerobic digestion of glycerol derived from biodiesel manufacturing. Bioresour. Technol. 2009, 100, 5609–5615.
- Liu, Y.; Koh, C.M.J.; Ji, L. Bioconversion of crude glycerol to glycolipids in Ustilago maydis. Bioresour. Technol. 2011, 102, 3927–3933.
- Suzuki, T.; Nishikawa, C.; Seta, K.; Shigeno, T.; Nakajima-Kambe, T. Ethanol production from glycerol-containing biodiesel waste by Klebsiella variicola shows maximum productivity under alkaline conditions. New Biotechnol. 2014, 31, 246–253.
- Maru, B.T.; López, F.; Kengen, S.W.M.; Constantí, M.; Medina, F. Dark fermentative hydrogen and ethanol production from biodiesel waste glycerol using a co-culture of Escherichia coli and Enterobacter sp. Fuel 2016, 186, 375–384.
- Rodrigues, C.V.; Santana, K.O.; Nespeca, M.G.; de Oliveira, J.E.; Maintinguer, S.I. Crude glycerol by transesterification process from used cooking oils: Characterization and potentialities on hydrogen bioproduction. Int. J. Hydrogen Energy 2016, 41, 14641–14651.
- Rodrigues, C.V.; Nespeca, M.G.; Sakamoto, I.K.; de Oliveira, J.E.; Amâncio Varesche, M.B.; Maintinguer, S.I. Bioconversion of crude glycerol from waste cooking oils into hydrogen by sub-tropical mixed and pure cultures. Int. J. Hydrogen Energy 2019, 44, 144–154.
- Yuwa-amornpitak, T.; Chookietwatana, K. Bioconversion of waste cooking oil glycerol from cabbage extract to lactic acid by Rhizopus microsporus. Braz. J. Microbiol. 2018, 49, 178–184.
- Wang, X.L.; Zhou, J.J.; Sun, Y.Q.; Xiu, Z.L. Bioconversion of raw glycerol from waste cooking-oil-based biodiesel production to 1,3-propanediol and lactate by a microbial consortium. Front. Bioeng. Biotechnol. 2019, 7, 14.
- Ma, J.; Jiang, H.; Hector, S.B.; Xiao, Z.; Li, J.; Liu, R.; Li, C.; Zeng, B.; Liu, G.-Q.; Zhu, Y. Adaptability of Klebsiella pneumoniae 2e, a newly isolated 1,3-propanediol-producing strain, to crude glycerol as revealed by genomic profiling. Appl. Environ. Microbiol. 2019, 85, e00254-19.
- Chen, J.; Yan, S.; Zhang, X.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Chemical and biological conversion of crude glycerol derived from waste cooking oil to biodiesel. Waste Manag. 2018, 71, 164–175.
- Xu, J.; Zhao, X.; Wang, W.; Du, W.; Liu, D. Microbial conversion of biodiesel byproduct glycerol to triacylglycerols by oleaginous yeast Rhodosporidium toruloides and the individual effect of some impurities on lipid production. Biochem. Eng. J. 2012, 65, 30–36.
- Guerfali, M.; Ayadi, I.; Sassi, H.E.; Belhassen, A.; Gargouri, A.; Belghith, H. Biodiesel-derived crude glycerol as alternative feedstock for single cell oil production by the oleaginous yeast Candida viwanathii Y-E4. Ind. Crops Prod. 2020, 145, 112103.
- Braga, A.; Freitas, B.; Cordeiro, A.; Belo, I. Valorization of crude glycerol as carbon source for the bioconversion of L-phenylamine to 2-penylethanol by Yarrowia species. J. Chem. Technol. Biotechnol. 2021, 96, 2940–2949.
- Ripoll, M.; Velasco-Lozano, S.; Jackson, E.; Diamanti, E.; Betancor, L.; López-Gallego, F. One-pot biotransformation of glycerol into serinol catalyzed by biocatalytic composites made of whole cells and immobilised enzymes. Green Chem. 2021, 23, 1140–1146.
- Dobson, R.; Gray, V.; Rumbold, K. Microbial utilization of crude glycerol for the production of value-added products. J. Ind. Microbiol. Biotechnol. 2012, 39, 217–226.
