Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Skin flaps are necessary in plastic and reconstructive surgery for the removal of skin cancer, wounds, and ulcers. A skin flap is a portion of skin with its own blood supply that is partially separated from its original position and moved from one place to another. The use of skin flaps is often accompanied by cell necrosis or apoptosis due to ischemia–reperfusion (I/R) injury.
- inflammatory cytokine
- skin flap animal model
- stem cell
- biomaterial
1. Introduction
Random pattern skin flaps are frequently used in plastic and reconstructive surgery to treat skin ulcers, trauma, congenital disease, general wounds, and wounds resulting from tumor excision [1][2]. However, skin flaps are often accompanied by necrosis or apoptosis via ischemia/reperfusion (I/R) injury that activates proinflammatory cytokines. These activated proinflammatory molecular factors accelerate a variety of factors such as cytokines, chemokines, adhesion, and inducible enzymes [3][4][5][6][7]. To prevent I/R injury after skin flap surgery, many investigators report various treatments. For example, prior researchers suggested the inhibition of proinflammatory cytokines including nuclear factor kappa B (NF-κB), Inhibitor of kappa B (IκB), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and oxygen free radicals [3][4][8]. Novel drugs that regulate proinflammatory cytokines are actively being studied [9][10]. Chehelcheraghi et al. investigated the effect of bone marrow mesenchymal-derived stem cells (BM-MSCs) on the viabilities of random pattern skin flap models [2]. Furthermore, Gersch et al. induced angiogenesis using vascular endothelial growth factor (VEGF) in mouse skin flap models [11].
Animal models of skin flaps are widely used in plastic and reconstructive surgery, as these animal models are of low cost and I/R injury is easily evaluated. The rectangular skin flap model was suggested by McFarlane et al. (McFarlane flap), and many researchers have modified this flap model for various experimental approaches [12][13]. Although a 27 cm2 (3 cm × 9 cm) dorsal skin flap was frequently used in rat skin flap models, various skin flap methods are utilized according to their purpose. It will introduce a variety of skin flap animal models and present future strategies by summarizing the latest research trends for skin flap treatment and ischemic preconditioning (IPC). Experimental animal models are generally standardized or modified according to the concept of research. However, regarding the case of skin flaps, too many modified animal models have been proposed based on the McFarlane flap or island flap. Therefore, it has been compiled various skin flap animal models so that researchers starting flap research can select an appropriate animal model. The skin flap animal model was organized by searching for published papers in the last ten years on PubMed stratified by type of animals such as rat, mouse, rabbit, and pig.
IPC is a non-invasive treatment method to prevent I/R injury. In previous ones, IPC was proposed as an effective method to minimize I/R injury by promoting angiogenesis in various organs [14]. However, IPC was also performed with various animals and procedures. It was investigated the IPC in the skin flap model from 2007 to 2021 through the PubMed search sorted by animal type, and IPC methods. In addition, the molecular factors related to IPC are organized so that researchers can easily understand and select the analysis target molecules of IPC. It was summarized the vast amount of data on skin flap animal models and IPCs, and also briefly presented trends and clinical treatments for skin flaps.
2. Animal Experimental Models for Skin Flaps
The skin flap is an important and frequently used tool in plastic and reconstructive surgery [2][15]. To reduce postoperative flap complications, a variety of strategies have been studied in skin flap animal models. McFarlane first presented the standardized dorsal rat skin flap models in 1965 [13]. The McFarlane flap (4 cm × 10 cm) is a widely known rectangular skin flap model that has been modified to create a variety of other skin flap models [12][13][16]. For experimental purposes, researchers should choose the most appropriate flap model. Below, It was introduced and summarize many of the various animal experimental models for skin flaps.
2.1. Animals and Flap Designs
With regards to rectangular skin flap models, most were modified McFarlane flaps, with the preferred flap being a 27 cm2 (3 cm × 9 cm) dorsal skin flap (Figure 1) [1][10][11][15][17][18][19][20][21][22][23][24][25][26][27][28][29]. In the operation, a 9 cm long red line originating at the level of the base of the scapulae was drawn on the dorsal midline. A rectangular area was drawn with its long edges parallel to and 1.5 cm away from the midline. The skin was incised along the cranial and lateral lines of the rectangular area. The skin flap was immediately re-attached in its original position and sutured using various sutures including 4-0 silk, 4-0 nylon, or 4-0 prolene single stitches at 0.5 cm intervals [13][15][18][19][20][21]. In addition, skin flap models vary according to the purpose, and 24–30 cm2 flaps are used in about 70% of the papers published recently [11][30][31][32][33][34][35][36]. A 9 cm2 (1.5 cm × 6 cm) flap was the smallest [37][38], and a 65 cm2 (5 cm × 13 cm) flap was the largest [11]. Most researchers used a dorsal flap model, but Bai et al. performed an abdominal skin flap (6 cm × 9 cm) with a surgical procedure similar to the method described above [39].
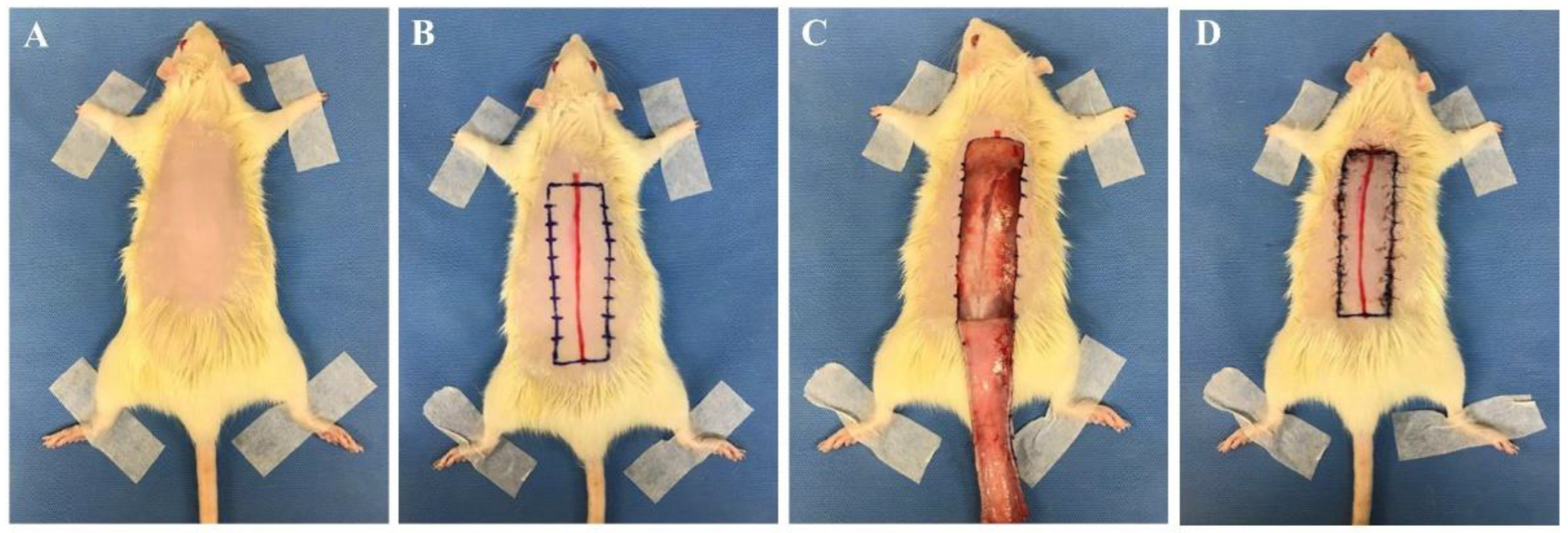
Figure 1. Skin flap procedure in rats. (A) Hair from the dorsal side of the rat was removed. (B) A 9 cm long red line originating at the level of the base of the scapulae was drawn on the dorsal midline. A rectangular area was drawn with its long edges parallel to and 1.5 cm away from the midline. (C) The skin was incised along the cranial and lateral lines of the rectangular area. (D) The skin flap was immediately re-attached in its original position and sutured with 4-0 nylon single stitches at 0.5 cm intervals. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Seoul National University Bundang Hospital (BA1612-213/075-01).
In addition to the McFarlane flap model, island flaps are also widely used as animal skin flap models. In 1982, Hartrampt et al. reported an island skin flap that could be harvested transversely across the lower abdomen [40]. Animal models of island skin flaps are continuously developed and used in various animal experiments and are representative of the epigastric vessel model. In the epigastric island flap operation, a rectangular area is marked on the abdomen, and the skin flap based on the right superficial epigastric vessel is elevated [3][41]. Island flap size varies from 9 to 54 cm2, being relatively smaller than the McFarlane flap [3][32][41][42][43].
Dorsal skin flap sizes in mice commonly range from 3 to 8 cm2 [44][45], and the rectangular dorsal flap method is similar to the method seen in rat models. Common island flaps are the dorsal lateral thoracic vessel (1.5 cm × 3.5 cm) [46] and epigastric vessel models [47]. Mouse strains used for skin flap procedures include C57BL/6, BALB/c, and ICR, and were selected according to research purpose.
The rabbit is mainly chosen for island flap research [48][49][50][51][52], but some researchers use rabbits as dorsal skin flap models [53][54]. Rabbit island flap models were commonly epigastric (5 cm × 17 cm) [51], fasciocutaneous (4 cm × 5 cm, 10 cm × 10 cm) [49][52], abdominal cutaneous (15 cm × 19 cm, 6-0 polypropylene sutures) [48], and artery graft flap models (12 cm × 13 cm) [50] . The flap of the island skin flap consists of the skin, subcutaneous tissue, and superficial fascia (or panniculus carnosus, etc.). The flap is marked based on the medial branch of the superficial inferior epigastric artery (or target vessel). After flap elevation, target research such as I/R injury or artery grafts are conducted. The island flap is immediately repositioned and sutured [48][52]. Zhuang et al. created a 15 cm2 (2.5 cm × 6 cm, 7-0 prolene suture) dorsal skin flap and Wang et al. created two 16 cm2 (2 cm × 8 cm, 5-0 monofilament nylon suture) flaps [53][54]. The rabbit dorsal skin flap surgical procedure is similar to that used in rat and mouse skin flap creation.
2.2. Skin Flap Evaluation
Skin flap survival is evaluated by a variety of methods including skin color measures, histopathologic assessment, immunohistochemistry, and inflammatory factor evaluation [2][3][4][11][15][17].
2.2.1. Necrosis Flap Area Analysis
The analysis of a necrosis flap area is widely used to evaluate flap survival. For quantitative evaluation of flap viability, the skin flap is photographed 7–8 days postoperatively. To measure the necrotic or apoptotic tissue, total skin flap, and necrotic areas are commonly measured using imaging analysis programs (e.g., Image J software, Adobe Photoshop CS6 extended software, and software Image-Pro Plus 6.0) [2][3][17]. The necrotic area presents with eschar formation and dark skin color when compared to the zero- or first-day postoperative appearance.
2.2.2. Histopathologic Assessment
The histopathologic approaches to detect necrosis and inflammation are important and reveal information such as granulation tissue quality, tissue edema, blood vessel and capillary hyperplasia, and inflammatory cell infiltration [15]. Skin flap animals are commonly sacrificed 7–8 days postoperatively, after which tissues are fixed, embedded, and serially sectioned. Most investigators perform hematoxylin and eosin staining for histopathologic assessment [3][15]. In a rat skin flap model, acute inflammatory infiltration was observed and, other than a portion of its muscle fibers, the epithelial layer was degenerated [20]. The vessel walls were sclerosed and had collapsed [55]. Moreover, inflammatory cells were observed in the dermal and subcutaneous layers [39]. Miyawaki et al. used a histopathologic scoring system based on inflammation, edema, and congestion [56].
2.2.3. Inflammatory Cytokines
The I/R injury induces expressions of various inflammatory cytokines and tissue damage [4][57]. The exploration of inflammatory cytokines plays a key role in improving flap survival and may provide evidence for clinical trials. The inflammatory cytokine pathway is a complex network including components such as NF-κB, IκB, IL-6, TNF-α, and oxygen free radicals [57].
-
NF-κB and IκB
-
NF-κB is a known transcription factor that controls cytokine expression and cell survival in normal cells [3][4]. In addition, NF-κB regulates chemokine, adhesion, and inducible enzymes [58]. NF-κB dimer (RelA/p50) binds to IκB and maintains an inactive form in the cytoplasm of most resting cells. In the condition of inflammatory stimulation, IκB kinase (IKK) induces IκB phosphorylation and degradation. NF-κB separates from the NF-κB/IκB complex, and the activated NF-κB dimer (RelA/p50) translocates to the nucleus. The NF-κB dimer (RelA/p50) binds to the promoter of pro-inflammatory genes in the nuclear DNA. Finally, pro-inflammatory transcription induces the expression of inflammatory cytokines such as TNF-α, IL-1, and IL-6 (Figure 2) [4][5][6][7]. Therefore, NF-κB signal regulation is important when attempting to improve I/R injury in the skin flap.
-
-
TNF-α, IL-1β, and IL-6
-
TNF-α, IL-1β, and IL-6 play key roles as proinflammatory cytokines in I/R injury [59][60]. As described above, proinflammatory cytokines are activated by NF-κB and used as indicators of inflammation. Prior investigators have researched the potential of these cytokines to improve skin flap survival or discover novel therapeutics.
-
TNF-α is a systemic inflammation cell signaling protein expressed by activated NF-κB via the PARs/p38-MAPK/NF-κB pathway [4]. It is released from activated monocytes and macrophages and can activate lymphocytes, neutrophils, eosinophils, and natural killer (NK) cells during an inflammatory response [9]. Moreover, increased TNF-α triggers additional NF-κB expressions via IKK activation [4]. Many investigators have attempted to inhibit TNF-α expression. Deheng et al. reported TNF-α presence and the inflammatory reactions were decreased by VEGF treatment, which improved skin flap survival [61].
-
Interleukin (IL) families play a key role in immune system regulation, and are synthesized by helper CD4 T lymphocytes, monocytes, macrophages, and endothelial cells [8]. In I/R injury, IL-1β and IL-6 are known as proinflammatory mediators produced by leukocytes. Increased TNF-α via the PARs/p38-MAPK/NF-κB pathway enhances the expression of IL-1β and IL-6. IL-1β is mainly secreted by activated immune cells such as monocytes and macrophages, as well as NK cells, B cells, dendritic cells, fibroblasts, and epithelial cells [8][62]. Alongside TNF-α and IL-1β, IL-6 also acts as an indicator of inflammation severity [4]. It is a pyrogen and responds to fever in autoimmune, infectious, or non-infectious diseases. In skin flap animal experiments, IL-1β and IL-6 are usually increased due to skin flap necrosis. Many investigators use the IL factors as inflammatory indicators after skin flap procedures. Peng et al. reported that natural hirudin treatment improved skin flap viability via inhibition of proinflammatory TNF-α and IL-6 [4]. Deheng et al. investigated the effect of salidroside on skin flap survival, and found that salidroside promoted VEGF expression, increased skin flap angiogenesis, and decreased the presence of proinflammatory cytokines [10]. As mentioned above, many researchers have an interest in new drugs to improve skin flap survival [10][15][42]. New drug development for the inhibition of inflammation will require continuous research.
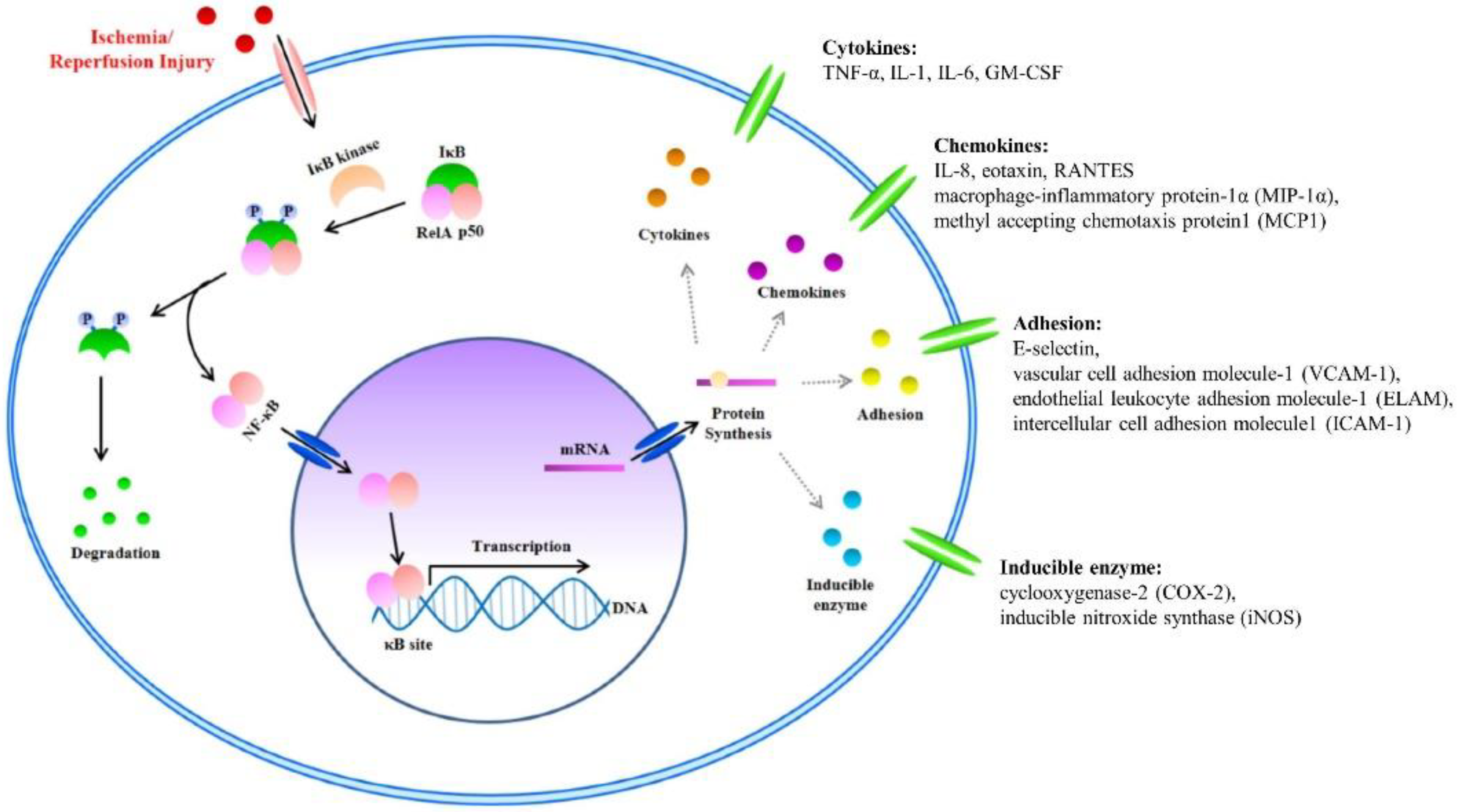
Figure 2. The nuclear factor kappa B (NF-κB) signal and Inflammatory Factors in I/R injury. I/R injury factors enter the cytoplasm. Activated inhibitor of kappa B (IκB) kinase separates the NF-κB/IκB complex into NF-κB and IκB. Separated IκB is degraded in the cytoplasm, and the NF-κB dimer (RelA/p50) translocates to the nucleus. Within the nucleus, the NF-κB dimer (RelA/p50) binds to the DNA promoter of pro-inflammatory genes. Finally, pro-inflammatory transcription induces the expression of inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1, and IL-6.
2.2.4. Apoptosis
Apoptosis is an important signal that frequently occurs in skin flaps. Inflammatory reactions and oxidative stress accelerate the apoptotic reaction [10]. To detect apoptosis in experimental ones, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining is performed. TUNEL-positive cell presence increases after skin flap creation [63]. B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X protein (Bax), phospho-apoptosis signal regulating kinase-1 (pASK-1), phospho-jun amino-terminal kinases (pJNK), and caspase-3 are important apoptosis signal factors experimentally detected by western blot or qPCR. Bax is a member of the Bcl-2 family and is associated with the apoptosis pathway [64]. It activates caspase-3 via the release of cytochrome c from mitochondria, and finally induces DNA fragmentation [65]. Bcl-2 is an anti-apoptosis protein and a mitochondrial anchoring protein [4][66]. It can regulate apoptosis via the mitochondrial pathway of apoptosis by regulating the ratio between anti-apoptotic and pro-apoptotic members of the Bcl-2 family [66]. According to prior one, Bax and caspase-3 presence increases, and Bcl-2 decreases after skin flap procedures [41]. For that reason, many researchers explore the apoptosis pathway to increase skin flap survival. Deheng et al. found that salidroside improved the area of skin flap survival. Furthermore, the expression of caspase-3 was decreased and Bax was increased in the salidroside-treatment group [10]. According to Almeida et al., hyperbaric oxygen therapy induces a reduction in cellular DNA damage and apoptosis [63]. To introduce therapeutics that prevent skin flap apoptosis, however, many additional are needed.
2.2.5. Angiogenesis
There is limited blood supply during skin flap transplantation, and the flap boundary far from the main blood vessels is easily necrotic after transplantation. For that reason, angiogenesis of the skin flap becomes the biggest problem to solve. Skin flap researchers have investigated various flap survival ones, mainly focusing on angiogenesis. Improving blood supply by increasing new blood vessel formation and establishing a new capillary network can improve the survival of the flap. Angiogenesis is a process of new blood vessel formation from the pre-existing vasculature, which mainly occurs when tissues need sufficient nutrients and oxygen supply [67]. It is regulated by various molecular pathways, including the hypoxia-inducible factor-1α (HIF-1α)/VEGF pathway [22]. HIF-1 is a critical nuclear transcriptional regulator that promotes angiogenesis and is an important target for a variety of therapies. Under hypoxic conditions, hydroxylation is inhibited and HIF-1α is accumulated. It induces transcription by interacting with hypoxia-response elements in the promoters of oxygen-sensitive genes such as VEGF, platelet-derived growth factor, and angiogenin [22]. Angiogenesis in skin flap animal models includes histopathological assessment (e.g., hematoxylin and eosin staining), immunohistochemical staining (for example, CD31 and von Willebrand factor), and protein and RNA expression of angiogenesis-related factors. The microvessel density is determined by the number of microvessels per unit area in randomly selected fields under light microscopy. The laser doppler flowmetry measures the capillary blood flow of the skin non-invasively, so it can continuously evaluate the survival of the skin flap without animal sacrifice [22]. The microvascular structure of the flap can be clearly seen in the X-ray image through systemic angiography [68].
It was found that several ones improved skin flap survival by promoting angiogenesis. Yu et al. reported that ADSCs improve flap survival by increasing the expression of HIF-1α and VEGF and inducing angiogenesis by regulating the HIF-1α/VEGF pathway [69]. VEGF administration includes the direct injection of exogenous VEGF into the skin flap end or gene therapy using viral vectors. Administration of exogenous VEGF or VEGF-viral vector to the flap significantly increased flap survival and blood vessel density, thereby improving the survival rate of the skin flap [70][71]. However, It was recognized that it is challenging to solve the side effect of skin flaps, suppress necrosis, and improve flap survival. Therefore, combining biomaterials or other treatment methods instead of applying a single substance such as exogenous VEGF injection seems more effective and promising. In particular, since angiogenesis is regulated by complex pathways and signals, more preclinical and clinical researches are needed to identify it.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23095234
References
- Kailiang, Z.; Yihui, Z.; Dingsheng, L.; Xianyao, T. Effects of Muscone on Random Skin Flap Survival in Rats. J. Reconstr. Microsurg. 2016, 32, 200–207.
- Chehelcheraghi, F.; Eimani, H.; Sadraie, S.H.; Torkaman, G.; Amini, A.; Shemshadi, H.; Majd, H.A. Improved viability of random pattern skin flaps with the use of bone marrow mesenchymal-derived stem cells and chicken embryo extract. Iran. J. Basic Med. Sci. 2015, 18, 764–772.
- Han, H.H.; Lim, Y.M.; Park, S.W.; Lee, S.J.; Rhie, J.W.; Lee, J.H. Improved skin flap survival in venous ischemia-reperfusion injury with the use of adipose-derived stem cells. Microsurgery 2015, 35, 645–652.
- Peng, L.; Pan, X.; Yin, G. Natural Hirudin Increases Rat Flap Viability by Anti-Inflammation via PARs/p38/NF-kappaB Pathway. Biomed. Res. Int. 2015, 2015, 597264.
- Liang, F.; Kang, N.; Liu, X.; Yang, J.; Li, Z.; Tan, J.W. Effect of HMGB1/NF-kappaB in hyperbaric oxygen treatment on decreasing injury caused by skin flap grafts in rats. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2010–2018.
- Kang, N.; Hai, Y.; Liang, F.; Gao, C.J.; Liu, X.H. Preconditioned hyperbaric oxygenation protects skin flap grafts in rats against ischemia/reperfusion injury. Mol. Med. Rep. 2014, 9, 2124–2130.
- Blackwell, T.S.; Christman, J.W. The role of nuclear factor-kappa B in cytokine gene regulation. Am. J. Respir. Cell Mol. Biol. 1997, 17, 3–9.
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491.
- Silva, J.J.; Pompeu, D.G.; Ximenes, N.C.; Duarte, A.S.; Gramosa, N.V.; Carvalho Kde, M.; Brito, G.A.; Guimaraes, S.B. Effects of Kaurenoic Acid and Arginine on Random Skin Flap Oxidative Stress, Inflammation, and Cytokines in Rats. Aesthetic Plast. Surg. 2015, 39, 971–977.
- Deheng, C.; Kailiang, Z.; Weidong, W.; Haiming, J.; Daoliang, X.; Ningyu, C.; Huazi, X. Salidroside Promotes Random Skin Flap Survival in Rats by Enhancing Angiogenesis and Inhibiting Apoptosis. J. Reconstr. Microsurg. 2016, 32, 580–586.
- Gersch, R.P.; Fourman, M.S.; Phillips, B.T.; Nasser, A.; McClain, S.A.; Khan, S.U.; Dagum, A.B.; Bui, D.T. AdVEGF-All6A+ Preconditioning of Murine Ischemic Skin Flaps Is Comparable to Surgical Delay. Plast. Reconstr. Surg. Glob. Open 2015, 3, e494.
- McFarlane, R.M.; Deyoung, G.; Henry, R.A. The Design of a Pedicle Flap in the Rat to Study Necrosis and Its Prevention. Plast. Reconstr. Surg. 1965, 35, 177–182.
- Camargo, C.P.; Margarido, N.F.; Guandelini, E.; Vieira, G.A.; Jacomo, A.L.; Gemperli, R. Description of a new experimental model skin flap for studying skin viability in rats. Acta Cir. Bras. 2014, 29, 166–170.
- Xue, J.; Zhu, K.; Cao, P.; Long, C.; Deng, Y.; Liu, T.; Yin, G.; Li, X.; Wang, Z. Ischemic preconditioning-induced protective effect for promoting angiogenesis in renal ischemia-reperfusion injury by regulating miR-376c-3p/HIF-1alpha/VEGF axis in male rats. Life Sci. 2022, 299, 120357.
- Lin, B.; Lin, Y.; Lin, D.; Cao, B. Effects of Bezafibrate on the Survival of Random Skin Flaps in Rats. J. Reconstr. Microsurg. 2016, 32, 395–401.
- Esteves, G.R.; Junior, I.E.; Masson, I.F.B.; Machado, A.F.P.; Oliveira, M.C.D.; Baldan, C.S.; Farcic, T.S.; Liebano, R.E.; Plapler, H. Photobiomodulation effect in tumoral necrosis factor-alpha(TNF-alpha) on the viability of random skin flap in rats. Lasers Med. Sci. 2022, 37, 1495–1501.
- Masaoka, K.; Asato, H.; Umekawa, K.; Imanishi, M.; Suzuki, A. Value of remote ischaemic preconditioning in rat dorsal skin flaps and clamping time. J. Plast. Surg. Hand Surg. 2016, 50, 107–110.
- Roh, T.S.; Jung, B.K.; Yun, I.; Lew, D.H.; Kim, Y.S. Effect of botulinum toxin A on vasoconstriction and sympathetic neurotransmitters in a murine random pattern skin flap model. Wound Repair Regen. 2017, 25, 75–85.
- Dingsheng, L.; Zengbing, L.; Dong, H. Favorable effects of progesterone on skin random flap survival in rats. Iran. J. Basic Med. Sci. 2016, 19, 1166–1170.
- Lv, Q.B.; Gao, X.; Lin, D.S.; Chen, Y.; Cao, B.; Zhou, K.L. Effects of diammonium glycyrrhizinate on random skin flap survival in rats: An experimental study. Biomed. Rep. 2016, 5, 383–389.
- Chen, G.J.; Chen, Y.H.; Yang, X.Q.; Li, Z.J. Nano-microcapsule basic fibroblast growth factor combined with hypoxia-inducible factor-1 improves random skin flap survival in rats. Mol. Med. Rep. 2016, 13, 1661–1666.
- Liu, Y.; Li, W.; Ma, X.; He, J.; Lin, Y.; Lin, D. Rivastigmine Regulates the HIF-1alpha/VEGF Signaling Pathway to Induce Angiogenesis and Improves the Survival of Random Flaps in Rats. Front. Pharmacol 2021, 12, 818907.
- Li, W.J.; Liu, Y.Y.; He, J.B.; Ma, X.Y.; Lin, Y.; Zheng, P.; Lin, D.S. Effect of paeoniflorin on distal survival of random flaps. Int. Immunopharmacol. 2022, 105, 108562.
- Pak, C.S.; Moon, S.Y.; Lee, Y.E.; Kang, H.J. Therapeutic Effects against Tissue Necrosis of Remote Ischemic Preconditioning Combined with Human Adipose-Derived Stem Cells in Random-Pattern Skin Flap Rat Models. J. Investig. Surg. 2021, 34, 1304–1311.
- Fan, W.; Liu, Z.; Chen, J.; Liu, S.; Chen, T.; Li, Z.; Lin, D. Effect of memantine on the survival of an ischemic random skin flap and the underlying mechanism. Biomed. Pharmacother. 2021, 143, 112163.
- Jaleel, Z.; Blasberg, E.; Troiano, C.; Montanaro, P.; Mazzilli, S.; Gertje, H.P.; Crossland, N.A.; Platt, M.; Spiegel, J. Association of vaping with decreased vascular endothelial growth factor expression and decreased microvessel density in cutaneous wound healing tissue in rats. Wound Repair Regen. 2021, 29, 1024–1034.
- Huang, T.; Shi, J.; Sang, K.; Yu, C.; Xie, Y.; Chen, H.; Jin, Z.; Yan, H.; Zhao, B. The effect of different modes of microneedling technique on random flap survival in rats. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 2768–2775.
- Ma, X.; Lin, Y.; Liu, Y.; Li, W.; He, J.; Fang, M.; Lin, D. Effects of Apigenin Treatment on Random Skin Flap Survival in Rats. Front. Pharmacol. 2021, 12, 625733.
- Luo, Z.; Bian, Y.; Zheng, G.; Wang, H.; Yan, B.; Su, W.; Dong, W.; Hu, Z.; Ding, J.; Wang, A.; et al. Chemically Modified SDF-1alpha mRNA Promotes Random Flap Survival by Activating the SDF-1alpha/CXCR4 Axis in Rats. Front. Cell Dev. Biol. 2021, 9, 623959.
- Pan, X.Y.; Peng, L.; Han, Z.Q.; Yin, G.Q.; Song, Y.K.; Huang, J. Hirudin promotes angiogenesis by modulating the cross-talk between p38 MAPK and ERK in rat ischemic skin flap tissue. Tissue Cell 2015, 47, 301–310.
- Koh, K.S.; Park, S.W.; Oh, T.S.; Choi, J.W. Flap preconditioning by pressure-controlled cupping in a rat model. J. Surg. Res. 2016, 204, 319–325.
- Kagaya, Y.; Ohura, N.; Kurita, M.; Takushima, A.; Harii, K. Examination of tissue oxygen saturation (StO2) changes associated with vascular pedicle occlusion in a rat Island flap model using near-Infrared spectroscopy. Microsurgery 2015, 35, 393–398.
- Wald, G.; Van, Y.V.; Towne, W.; Otterburn, D.M. The Effect of Topical Tacrolimus on Pedicled Flap Survival: A Histological Analysis. Ann. Plast. Surg. 2021, 87, S57–S59.
- Khavanin, N.; Darrach, H.; Kraenzlin, F.; Yesantharao, P.S.; Sacks, J.M. The Intra.Ox Near-Infrared Spectrometer Measures Variations in Flap Oxygenation That Correlate to Flap Necrosis in a Preclinical Rodent Model. Plast. Reconstr. Surg. 2021, 147, 1097–1104.
- Dogan, R.; Metin Guler, E.; Kocyigit, A.; Bayindir, N.; Esrefoglu, M.; Mirasoglu, B.O.; Yenigun, A.; Ozturan, O. Comparison of the efficacy of multiple antioxidant and hyperbaric oxygen treatments in the prevention of ischemia and necrosis of local random McFarlane skin flap. J. Tissue Viability 2021, 30, 196–206.
- Nakagawa, T.; Sasaki, M.; Kataoka-Sasaki, Y.; Yotsuyanagi, T.; Radtke, C.; Kocsis, J.D.; Honmou, O. Intravenous Infusion of Mesenchymal Stem Cells Promotes the Survival of Random Pattern Flaps in Rats. Plast. Reconstr. Surg. 2021, 148, 799–807.
- Park, T.H.; Park, Y.J. The Effect of Botulinum Toxin A on Ischemia-Reperfusion Injury in a Rat Model. Biomed. Res. Int. 2017, 2017, 1074178.
- Kanayama, K.; Mineda, K.; Mashiko, T.; Wu, S.H.; Feng, J.; Kinoshita, K.; Sunaga, A.; Yoshimura, K. Blood Congestion Can Be Rescued by Hemodilution in a Random-Pattern Skin Flap. Plast. Reconstr. Surg. 2017, 139, 365–374.
- Bai, M.; Liu, Y.; Yin, D.; Zhang, M.; Wang, Y.; Ma, X.; Liu, Y.; Zhao, P. Inhibition of c-Jun N-terminal kinase signaling suppresses skin flap apoptosis in a rat ischemia and/or reperfusion model. J. Surg. Res. 2016, 206, 337–346.
- Hartrampf, C.R.; Scheflan, M.; Black, P.W. Breast reconstruction with a transverse abdominal island flap. Plast. Reconstr. Surg. 1982, 69, 216–225.
- Song, K.; Zhang, M.; Hu, J.; Liu, Y.; Liu, Y.; Wang, Y.; Ma, X. Methane-rich saline attenuates ischemia/reperfusion injury of abdominal skin flaps in rats via regulating apoptosis level. BMC Surg. 2015, 15, 92.
- Yue, Z.S.; Zeng, L.R.; Quan, R.F.; Tang, Y.H.; Zheng, W.J.; Qu, G.; Xu, C.D.; Zhu, F.B.; Huang, Z.M. 4Phenylbutyrate protects rat skin flaps against ischemiareperfusion injury and apoptosis by inhibiting endoplasmic reticulum stress. Mol. Med. Rep. 2016, 13, 1227–1233.
- Odake, K.; Tsujii, M.; Iino, T.; Chiba, K.; Kataoka, T.; Sudo, A. Febuxostat treatment attenuates oxidative stress and inflammation due to ischemia-reperfusion injury through the necrotic pathway in skin flap of animal model. Free Radic. Biol. Med. 2021, 177, 238–246.
- Fukunaga, Y.; Izawa-Ishizawa, Y.; Horinouchi, Y.; Sairyo, E.; Ikeda, Y.; Ishizawa, K.; Tsuchiya, K.; Abe, Y.; Hashimoto, I.; Tamaki, T. Topical application of nitrosonifedipine, a novel radical scavenger, ameliorates ischemic skin flap necrosis in a mouse model. Wound Repair Regen. 2017, 25, 217–223.
- Park, I.S.; Chung, P.S.; Ahn, J.C.; Leproux, A. Human adipose-derived stem cell spheroid treated with photobiomodulation irradiation accelerates tissue regeneration in mouse model of skin flap ischemia. Lasers Med. Sci. 2017, 32, 1737–1746.
- Rah, D.K.; Min, H.J.; Kim, Y.W.; Cheon, Y.W. Effect of Platelet-Rich Plasma on Ischemia-Reperfusion Injury in a Skin Flap Mouse Model. Int. J. Med. Sci. 2017, 14, 829–839.
- Tang, Y.H.; Pennington, L.A.; Scordino, J.W.; Alexander, J.S.; Lian, T. Dynamics of early stem cell recruitment in skin flaps subjected to ischemia reperfusion injury. Pathophysiology 2016, 23, 221–228.
- Huang, L. What happened if various kinds of postconditioning working on the preconditioned ischemic skin flaps. PLoS ONE 2013, 8, e72818.
- Prasetyono, T.O.; Adianto, S. The Relationship between Oxygen Saturation and Color Alteration of a Compromised Skin Flap: Experimental Study on the Rabbit. Arch. Plast. Surg. 2013, 40, 505–509.
- Yan, H.; He, Z.; Li, Z.; Lin, K.; Lv, L.; Li, Z.; Chen, X.; Gao, W. Large prefabricated skin flaps based on the venous system in rabbits: A preliminary study. Plast. Reconstr. Surg. 2013, 132, 372e–380e.
- Abe, Y.; Hashimoto, I.; Goishi, K.; Kashiwagi, K.; Yamano, M.; Nakanishi, H. Transcutaneous PCO2 Measurement at Low Temperature for Reliable and Continuous Free Flap Monitoring: Experimental and Clinical Study. Plast. Reconstr. Surg. Glob. Open 2013, 1, 1–8.
- Kim, H.Y.; Park, J.H.; Han, Y.S.; Kim, H. The effect of platelet-rich plasma on flap survival in random extension of an axial pattern flap in rabbits. Plast. Reconstr. Surg. 2013, 132, 85–92.
- Zhuang, Y.; Yang, M.; Liu, C. An Islanded Rabbit Auricular Skin Flap Model of Hyaluronic Acid Injection-Induced Embolism. Aesthetic. Plast. Surg. 2016, 40, 421–427.
- Wang, B.; Geng, Q.; Hu, J.; Shao, J.; Ruan, J.; Zheng, J. Platelet-rich plasma reduces skin flap inflammatory cells infiltration and improves survival rates through induction of angiogenesis: An experiment in rabbits. J. Plast. Surg. Hand Surg. 2016, 50, 239–245.
- Menevse, G.T.; TeomanTellioglu, A.; Altuntas, N.; Comert, A.; Tekdemir, I. Polidocanol injection for chemical delay and its effect on the survival of rat dorsal skin flaps. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 851–856.
- Miyawaki, T.; Jackson, I.T.; Elmazar, H.; Bier, U.C.; Barakat, K.; Andrus, L.; Williams, F. The effect of low-molecular-weight heparin in the survival of a rabbit congested skin flap. Plast. Reconstr. Surg. 2002, 109, 1994–1999.
- Zhang, F.; Hu, E.C.; Topp, S.; Lei, M.; Chen, W.; Lineaweaver, W.C. Proinflammatory cytokines gene expression in skin flaps with arterial and venous ischemia in rats. J. Reconstr. Microsurg. 2006, 22, 641–647.
- Wu, X.; Yu, M.; Li, A. Protective effect of a nuclear factor-kappaB inhibitor on ischemia-reperfusion injury in a rat epigastric flap model. J. Reconstr. Microsurg. 2008, 24, 351–359.
- Bennett, N.T.; Schultz, G.S. Growth factors and wound healing: Biochemical properties of growth factors and their receptors. Am. J. Surg. 1993, 165, 728–737.
- Gailit, J.; Clark, R.A. Wound repair in the context of extracellular matrix. Curr. Opin. Cell Biol. 1994, 6, 717–725.
- Fang, T.; Lineaweaver, W.C.; Chen, M.B.; Kisner, C.; Zhang, F. Effects of vascular endothelial growth factor on survival of surgical flaps: A review of experimental studies. J. Reconstr. Microsurg. 2014, 30, 1–13.
- Stanley, A.C.; Wong, C.X.; Micaroni, M.; Venturato, J.; Khromykh, T.; Stow, J.L.; Lacy, P. The Rho GTPase Rac1 is required for recycling endosome-mediated secretion of TNF in macrophages. Immunol. Cell Biol. 2014, 92, 275–286.
- Almeida, K.G.; Oliveira, R.J.; Dourado, D.M.; Filho, E.A.; Fernandes, W.S.; Souza, A.S.; Araujo, F.H. Morphological study of rat skin flaps treated with subcutaneous dimethyl sulfoxide combined with hyperbaric oxygen therapy. Genet. Mol. Res. 2015, 14, 18160–18171.
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80.
- Ramadan, M.A.; Shawkey, A.E.; Rabeh, M.A.; Abdellatif, A.O. Expression of P53, BAX, and BCL-2 in human malignant melanoma and squamous cell carcinoma cells after tea tree oil treatment in vitro. Cytotechnology 2019, 71, 461–473.
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 1998, 3, 697–707.
- Rouwkema, J.; Khademhosseini, A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 2016, 34, 733–745.
- Tu, Q.; Liu, S.; Chen, T.; Li, Z.; Lin, D. Effects of adiponectin on random pattern skin flap survival in rats. Int. Immunopharmacol. 2019, 76, 105875.
- Yu, W.Y.; Sun, W.; Yu, D.J.; Zhao, T.L.; Wu, L.J.; Zhuang, H.R. Adipose-derived stem cells improve neovascularization in ischemic flaps in diabetic mellitus through HIF-1alpha/VEGF pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 10–16.
- Vourtsis, S.A.; Spyriounis, P.K.; Agrogiannis, G.D.; Ionac, M.; Papalois, A.E. VEGF application on rat skin flap survival. J. Investig. Surg. 2012, 25, 14–19.
- Huang, N.; Khan, A.; Ashrafpour, H.; Neligan, P.C.; Forrest, C.R.; Kontos, C.D.; Pang, C.Y. Efficacy and mechanism of adenovirus-mediated VEGF-165 gene therapy for augmentation of skin flap viability. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H127–H137.
This entry is offline, you can click here to edit this entry!
