Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Chemical
In addition to acetoin and 2,3-butanediol, another class of compounds that is readily derivable from biochemical conversions is C4 dicarboxylic acids; specifically, these are succinic (butanedioic acid), fumaric (trans-butenedioic acid), and malic (hydroxybutanedioic acid) acids.
- sugarcane
- sugar beet
- fermentation
- biorefinery
1. Introduction
Among the greatest challenges at present in chemical engineering research and development is the need to supplant fossil fuels and petrochemicals with renewable biomass-derived analogues. This issue is centered largely on the non-renewable nature of fossil resources and their deleterious effects on the environment, including climate change [1,2]. Because of the persistent need for carbonaceous fuels, chemicals and materials in the global economy, biomass is the only viable sustainable feedstock with the potential for carbon-neutral production [2,3,4,5]. In general, a major contributor to the final cost for a biomass-derived product is typically the cost of the initial biomass feedstock (particularly on a dry, ash-free basis) [6,7,8,9]. To overcome this economically driven obstacle, the effective utilization of biomass-derived wastes and by-products is critical. Using wastes is desirable because they are abundantly available at low cost, with the intrinsic benefit of affording new waste management opportunities [10,11].
A biomass sector with significant potential for greater by-product utilization is agricultural production of sugar crops, including sugarcane and sugar beets [12]. Global sugar (i.e., sucrose) production is nearly 200 million tons per year, of which 75–80% comes from sugarcane and 20–25% comes from sugar beets [13,14]. For a given quantity of sugarcane, a larger mass of waste and by-products is generated than the total mass of final sugar produced. Meghana and Shastri report that for the production of 100 kg of sugar from 1000 kg of cane, a total of 300 kg bagasse and 40 kg of molasses are generated [13]. Lignocellulosic bagasse residues are typically consumed for energy to be used in sugar and ethanol plants, however, large surpluses often remain [15]. Sugarcane molasses is made up of a significant amount of fermentable sugars, containing roughly 30–35% sucrose and 10–25% glucose and fructose according to one estimate [16]. Roughly 30 kg of press mud (itself containing 5–10% sugar) per 100 kg of sugar (from 1000 kg cane) are also generated [13,16]. The processing of sugar beets for sucrose production also yields large quantities of lignocellulosic sugar beet pulp as a primary by-product. From 1000 kg of sugar beets (with comparable sucrose yields to sugarcane), approximately 70 kg of dried pulp (250 kg wet basis) are produced [17,18]. Sucrose production from sugar beets, like sugarcane, also generates sugar beet molasses containing approximately 50% sugar. Sugar beet molasses is typically used in fermentations for alcohol production and in animal feed or fertilizer [19]. Numerous studies have reported on successful inclusion of sugar crop processing products and by-products in biochemical conversions to various products [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Further reading on sugarcane and sugar beet industries, emphasizing waste valorization, is available in recent reviews from Meghana and Shastri [13] and Rajaeifar et al. [37].
2. Microbial Production of C4 Dicarboxylic Acids
In addition to acetoin and 2,3-butanediol, another class of compounds that is readily derivable from biochemical conversions is C4 dicarboxylic acids; specifically, these are succinic (butanedioic acid), fumaric (trans-butenedioic acid), and malic (hydroxybutanedioic acid) acids. In order to make bio-based production of high value C4 dicarboxylic acids competitive and more economically feasible compared to fossil fuel-based production, it is imperative that microbial conversion of inexpensive biomass be optimized. Part of this process may involve metabolic engineering of strains, adaptation to inexpensive feedstocks, exporter engineering, and process development which includes optimization of aeration, pH, temperature, and isolation/purification steps. Microbial production of valuable, bio-based C4 dicarboxylic acids such as malic acid and succinic acid is performed primarily by filamentous fungi and anaerobic bacteria as well as yeast such as S. cerevisiae. In S. cerevisiae, the C4 dicarboxylic acids can be produced either in the cytosol by the reductive TCA branch or through modification of the mitochondrial TCA cycle [97]. Production strategies of C4 dicarboxylic acids in S. cerevisiae have been recently reviewed [97].
2.1. Exporter Engineering and Metabolic Engineering
One facet of engineering microbes to produce C4 dicarboxylic acids at a higher level involves ensuring efficient export to the cell surface through the process of exporter engineering. Keeping intracellular organic acid levels low circumvents feedback inhibition, toxic buildup, or utilization by another pathway within the cell. This strategy also facilitates easier isolation and purification from the fermentation medium. Multiple studies have implemented the heterologous expression of exporters or permeases belonging to different protein families in bacteria and fungi [75,98,99,100].
For instance, the Schizosaccharomyces pombe transporter Mae1, a member of the voltage-dependent slow-anion channel transporter (SLAC1) protein transporter family, has been found to transport succinic, malic and fumaric acids out of the cell when expressed in Xenopus oocytes. SLAC1 transporters contain two highly conserved phenylalanine residues in the transport channel involved in transport activity. Several Mae1, SLAC1 homologs, from fungal sources have been evaluated in S. cerevisiae through heterologous expression and were found to increase malate export. The SLAC transporter, Dct, from Aspergillus carbonarius (AcDct) increased malate secretion in yeast by 12-fold under neutral pH while the S. pombe Mae1 (SpMae1) expressed in yeast increased titers of succinic, malic, and fumaric acids by 3-, 8-, and 5-fold, respectively [98,99,101]. Since these SLAC transporters are independent of proton- or sodium-motive forces, this transport mechanism requires less energy than others, thereby enabling improved yields.
Additionally, transporter engineering is usually coupled with metabolic modifications. The creation of anapleurotic pathways is sometimes central to optimizing microbial production of downstream products. Several studies have reported efforts to enhance production of microbial pathways through the reductive and oxidative TCA pathways (Figure 3), which, combined with other strategies including exporter engineering, can significantly increase C4 dicarboxylic acid titer. Fumarate is between malate and succinate in the TCA cycle, and subsequently, emphasis is given to the microbial production of malic and succinic acids in the following sections.
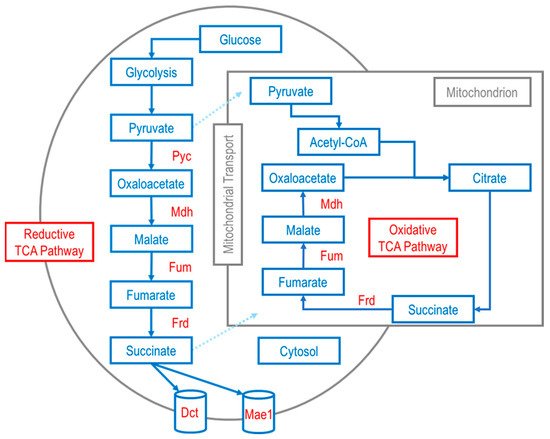
Figure 3. Example schematic of metabolic pathway(s) for C4 dicarboxylic acid production (succinate/succinic acid; fumarate/fumaric acid; malate/malic acid) in eukaryotes via the oxidative TCA pathway in the mitochondrion and the reductive TCA pathway in the cytosol. Enzymes of the depicted oxidative and reductive TCA pathways: pyruvate carboxylase (Pyc); malate dehydrogenase (Mdh); fumarase (Fum); fumarate reductase (Frd). Transport of C4 dicarboxylic acids out of the cytosol is aided by voltage-dependent slow-anion channel transporter (SLAC1) proteins transporters such as Mae1 and Dct. Adapted from previously published work [98,99,101].
2.2. Malic Acid
Aspergillus oryzae is a natural producer of malic acid and has been investigated for further optimization [102]. For instance, Liu et al. employed three strategic steps to dramatically improve L-malate titer in A. oryzae. First, they enhanced the reductive TCA (rTCA) pathway by overexpression of endogenous pyruvate carboxylase and malate dehydrogenase to drive carbon flux toward the rTCA pathway and improve malate titer. Next, an anapleurotic pathway to oxaloacetate was achieved by heterologous expression of E. coli-derived phosphoenol pyruvate caboxykinase and phosphoenol pyruvate carboxylase, which further increased malate titer. Third, to improve export from the cell and block malate transport back into the mitochondrial TCA cycle, they overexpressed the native C4-dicarboxylate transporter in A. oryzae and the S. pombe malate permease, Mae1. Additionally, the final strategy implemented was the identification of the potential rate limitation by 6-phosphofructokinase, which was overexpressed to further improve malate titer from 26.1 g/L in the parental strain to 93.2 g/L in flask culture and an impressive 165 g/L in fed-batch fermentation [103]. In another study, malic acid was produced by A. oryzae grown on lignocellulosic-derived acetate as an alternative carbon source, but much lower malic acid titers were achieved [104].
Aspergillus niger tolerates low pH and grows on a variety of renewable carbon sources, making this organism especially attractive for cost-effective fermentations. Recently, a Cre-loxP-based genetic system was developed in A. niger to construct “A. niger cell factories” that produce high levels of organic acids [105]. The deletion of oahA was found to block the oxalic acid biosynthesis pathway, allowing carbon flux from oxaloacetate to flow toward a higher production of malic acid instead. Furthermore, the expressions of pyruvate carboxylase (Pyc) and malate dehydrogenase (Mdh3) were used to enhance the rTCA cycle along with expression of a C4 dicarboxylic acid transporter c4t318 from A. oryzae. This strategy increased malic acid via the rTCA pathway to 120.38 g/L in flask fermentations and 201.24 g/L during fed batch fermentation [105].
2.3. Succinic Acid
Some natural producers of succinic acid include Mannheimia succiniciproducens, Actinobacillus succinogenes, Anaerobiospirillum succiniciproducens, and Basfia succiniciproducens [102,106]. For instance, a wild type strain of A. succinogenes was reported to produce 67.2 g/L succinic acid during batch anaerobic fermentation on glucose with a productivity of 0.8 g/L/h [107]. Another study used immobilized A. succinogenes cultures entrapped within alginate beads in a three-phase fluidized reactor to produce 31 g/L succinic acid with 35.6 g/L/h productivity [108]. Moreover, other strategies such as dual-phase fed batch fermentation have been reported for C. glutamicum during which the first phase involves cell growth to optimal OD followed by succinic acid production in the second phase [109]. Besides natural succinic acid producers, other studies have reported engineered bacterial strains that produce high levels of succinic acid including E. coli [110,111,112].
Further optimization of natural succinic acid producers has also led to improved succinic acid production. Basfia succiniciproducens was optimized to produce 20 g/L succinic acid by deleting pflD and ldhA, to eliminate formic acid and reduce lactic acid production resulting in increased carbon flux toward pyruvic acid and succinic acid [113,114]. Furthermore, 16s rRNA analysis indicates that B. succiniciproducens is very closely related to M. succiniciproducens.
The M. succiniciproducens strain MBEL55E was originally isolated from the rumen of a Korean cow and found to produce high levels of succinic acid [115]. In the capnophilic M. succiniciproducens, the formation of succinic acid involves carboxylation of phosphoenol pyruvate (PEP) by either PEP carboxykinase or PEP carboxylase to oxaloacetate during anaerobic respiration in the presence of CO2 [116]. PEP carboxylation flux is decreased when CO2 is replaced with N2, but when H2 is added to the fermentation in the presence of CO2, succinic acid levels increased, likely due to additional reducing power [117]. Increased flux toward oxaloacetate flows into the reductive TCA cycle which proceeds through malate, and fumarate as the terminal electron acceptor resulting in the formation of succinate. Furthermore, one patent reported fermentation with M. succiniciproducens using glycerol and sucrose as carbon sources to produce succinic acid with high productivity of 29.7 g/L/h [106,118]. Another study reported using a PALFK strain that was constructed with deletions in ldhA, pta, ackA, and fruA to produce 78.4 g/L homo-succinic acid with a productivity of 6.02 g/L/h [119].
Metabolic flux analysis based on updated genome metabolic information of pathways, metabolites, and gene deletions has been performed to optimize and balance cell growth rate with succinic acid production rate [120,121,122]. The M. succiniciproducens PALK strain was generated from the LK strain background containing a lactate dehydrogenase disruption ΔldhA. Additional deletions in pta (phosphotransacetylase) and ackA acetate kinase were performed to dramatically reduce acetic acid and lactic acid byproduct formation, direct carbon flux toward succinic acid formation, and simplify recovery and purification efforts thereby lowering costs [122]. Even without the pflB deletion, no formic acid was formed, and pyruvic acid was the main byproduct. Pyruvic acid accumulation was ameliorated through implementation of chemically defined medium, which resulted in 66.14 g/L during fed-batch fermentation. Titer was further improved by pH control measures with magnesium hydroxide and ammonia, which improved succinic acid titer to 90.68 g/L underscoring the impact of optimized pH control [122].
One study reported using elementary mode analysis with clustering to examine the M. succiniciproducens metabolic networks and predicted that overexpression of the zwf gene would increase succinic acid production [123]. The overexpression of zwf increased the NADPH levels that could be utilized by NADPH-dependent Arabidopsis thaliana malate dehydrogenase (Mdh) in the previously constructed LPK7 strain with deletions in ldhA, pflB, pta, and ackA that increase carbon flux to succinic acid [123]. The overexpression of both zwf and mdh revealed possible synergistic activity that resulted in improved succinic acid production.
In another study, high level succinate production was achieved by M. succiniciproducens heterologously expressing Corynebacterium glutamicum malate dehydrogenase (Mdh) with a higher specific activity for oxaloacetate reduction to malate and lower substrate inhibition than the endogenous MsMdh [124]. Fermentation with a high-inoculum, glycerol-glucose dual fed-batch fermentation yielded 134.25 g/L succinic acid and astonishing productivity of 21.1 g/L/h [125].
In addition, filamentous fungi such as Aspergillus sp. have been widely adapted and utilized for fermentation on various plant biomass feedstocks as microbial cell factories to produce organic acids [126]. The heterologous expression of A succinogenes phosphoenolpyruvate carboxykinase (AsPEPCK) and E. coli phosphoenolpyruvate carboxylase (EcPPC) in A. carbonarius has been used to direct enhanced carbon flux toward oxaloacetate and the reductive TCA pathway [127]. The later efforts of Yang et al. in A. carbonarius involved overexpression of the C4-dicarboxylate transporter Dct as well as heterologous expression of NADH-dependent fumarate reductase (Frd) from Trypanasoma brucei on a glucose oxidase-deficient parental strain background (Δgox) [99,128]. The Δgox genotype prevented conversion to gluconic acid, while Frd increased reduction in fumarate to succinate. The results showed a significant improvement in malic acid production and slight increase in succinic acid production with Dct overexpression, whereas the effect of Dct and Frd overexpression together significantly increased both malic acid and succinic acid levels (maximum reported titers of 32 g/L malic acid and 16 g/L succinic acid). Moreover, these results were obtained on wheat straw hydrolysate rich in both glucose and xylose, emphasizing the feasibility of organic acid production on renewable feedstocks [99].
More recently Yang et al. performed metabolic engineering in Aspergillus niger using ribonuceoprotein-based CRISPR-Cas9 technology to overcome low homologous recombination challenges. This approach enabled gene mutations in glucose oxidase (gox) and oxaloacetate hydrolase (oah) through non-homologous end joining and facilitated insertion of overexpression gene constructs for the A. carbonarius AcDct transporter and the NADH-dependent fumarate reductase (Frd) into the genome [100,128]. The highest titer of succinic acid achieved by the resulting SAP-3 strain was (17 g/L) after three days at 35 °C. The resulting SAP-3 strain was also able to utilize sugar beet molasses and wheat straw hydrolysate as inexpensive carbon sources, resulting in succinic acid titers of 23 g/L and 9 g/L after 6 days.
2.4. Co-Production Strategies during Fermentation
Alternatively, some strains have been modified to co-produce two valuable chemicals during fermentation. For example, Enterobacter cloacae was engineered to produce both acetoin and succinic acid by deletion of budC to block 2,3-BDO production and deletion of ldhA to block lactic acid production thereby improving carbon flux toward acetoin and succinic acid [129]. Co-production of 2,3-BDO and succinic acid has also been reported for E. cloacae [130]. In Propionibacterium acidipropionici, propionic acid and succinic acid were co-produced by semi-continuous fermentation where propionic acid was removed by membrane separation and chromatography to negate end-product feedback inhibition [131]. Moreover, a co-fermentation method for S. cerevisiae and A. succinogenes was recently reported for the co-production of succinic acid and ethanol [26]. S. cerevisiae fermentation utilizing hydrolyzed lignocellulosic biomass produced ethanol and CO2. The CO2 was then assimilated by A. succinogenes to produce succinic acid [26]. Another group reported high-level production of ethanol and succinic acid on a similar substrate using a robust S. cerevisiae strain [132]. During the S. cerevisiae fermentation, the CO2 produced during ethanol production was utilized to produce succinic acid via the reductive TCA pathway [133]. Another approach for co-production of 2,3-BDO and succinic acid by Klebsiella pneumoniae was to optimize pH and increase dissolved CO2 levels in fermentation medium for improved succinic acid yield [134]. Optimized CO2 levels have also been reported to improve succinic acid titers in Actinobacillus succinogenes fermentations [135]. Likewise, another K. pneumoniae strain, DSMZ2026, was reported to produce optimal levels of both 1,3-propanediol and 2,3-BDO during an anaerobic fermentation on glycerol when pH in the bioreactor was controlled and maintained at 7 [136].
2.5. Summary of Optimization Strategies for C4 Dicarboxylic Acids
Collectively, a diverse variety of strategies have been implemented to augment C4 dicarboxylic acid production, consideration of which may benefit and improve aspects of future studies. These include optimization of fermentation conditions including growth medium, growth rate, carbon source, pH control, O2, CO2, N2, and H2 levels [135]. In particular, the growth medium and method of carbon source feeding, whether in batch flask or fed-batch fermentations, can impact production. The sourcing of feedstock for carbon sources in the growth medium not only impacts growth and production of dicarboxylic acids, but also affects whether the microbial production is cost effective. Additionally, in some cases, bioreactor conditions such as pH determine whether the dicarboxylic acid or conjugate base is formed, as is the case with succinic acid or succinate. Additionally, specialized bioreactor conditions may also involve immobilization of cultures by adherence or entrapment [108]. Co-fermentation strategies that produce two high value chemicals in the same bioreactor with two different microorganisms can increase cost-effectiveness and overall productivity [129,130]. Another facet of engineering involves bioinformatic approaches that are informed by genomic data to conduct metabolic flux analysis, which considers pathways, genes, and metabolites and can further inform metabolic engineering approaches by predicting favorable genetic manipulations. [122,123].
Metabolic engineering strategies often involve the elimination of byproducts and unwanted anapleurotic pathways through targeted gene deletions. In addition, the heterologous expression of C4 dicarboxylic acid transporters can alleviate feedback inhibition and prevent utilization of accumulated end products by other pathways [75,98,99,100]. These strategies can also be paired with the creation of biosynthetic pathways in an engineered organism to create cell factories that are specifically tailored to produce the desired end product [97,100,128].
This entry is adapted from the peer-reviewed paper 10.3390/fermentation8050216
This entry is offline, you can click here to edit this entry!
