1. Introduction
In 2019, pneumonia of an unknown origin was reported in Wuhan, Hubei province of China, which was a tocsin to warn of a pandemic. The pneumonia cases were pathologically linked to a coronavirus, a novel beta-coronavirus belonging to the subgenus Sarbecovirus and was designated as ‘severe acute respiratory syndrome coronavirus 2’ (SARS-CoV-2) [
1]. Coronavirus disease 2019 (COVID-19) has since spread worldwide, pressing the World Health Organization to declare it as a pandemic on 12 March 2020 [
2]. The symptoms of COVID-19 range from mild to severe, while a large portion of infected people remain asymptomatic or mildly symptomatic carriers. The risk and severity of infection is higher in individuals with comorbidities such as diabetes, obesity, hypertension, cardiovascular and respiratory diseases [
3]. Generally, COVID-19 is characterized by fever, tiredness, cough, and other respiratory and gastrointestinal issues [
4]. The rapid transmissibility and wider community spread of COVID-19 may be attributed to the uncontrolled replication of SARS-CoV-2, the higher binding efficiency of its spike protein to human cell receptors, and its efficiency in overruling the host immune defense [
5,
6]. Although the major target of SARS-CoV-2 is the lungs, its systemic effects may lead to devastating effects on other vital organs such as the kidney, heart, liver, eye, and brain [
7]. To date, SARS-CoV-2 has affected over 200 countries and territories, causing several millions of deaths worldwide [
8].
Considerable time and effort were spent tracking, isolating, and treating COVID-19 patients in the early stages of the pandemic, which piled up an irrevocable socio-economic burden on several countries worldwide [
9]. Vaccines, antiviral drugs, and repurposed drugs continue to be used with different levels of efficacy to combat the new variants of SARS-CoV-2 emerging every few months [
10,
11,
12,
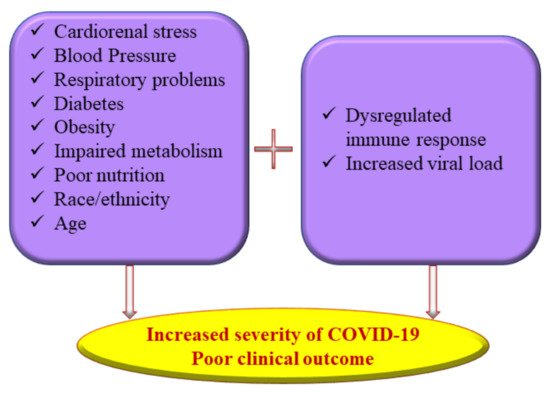
13]. The current research approach has seen a surge in interest in identifying the epidemiological factors underlying the susceptibility to COVID-19 and to exploring novel preventive and curative options. As the pandemic unfolds, it is evident that several physiological factors, including malnutrition, exacerbate the susceptibility and severity of coronavirus infections similar to any other viral disease (
Figure 1).
Figure 1. Multiple risk factors of severe coronavirus disease 2019 (COVID-19) illness.
2. Nutrition and COVID-19
Malnutrition is a state of nutrient inadequacy attributed to insufficient energy and health issues and is usually associated with multiple micronutrient deficiencies. Nutritional supplementation may reduce the susceptibility to infection, prevent severe disease, and help patients recover faster with milder post-recovery morbidities [
14]. Micronutrients such as vitamins and trace elements such as iron, zinc, copper, selenium, and omega-3-polyunsaturated fatty acids are essential for the healthy functioning of the immune system [
15].
Micronutrient deficiencies or suboptimal status may weaken immune responses and decrease resistance to infections. A study conducted in COVID-19 patients of China showed that poor nutritional status is a predisposing factor leading to higher disease severity. The prognostic nutritional index (PNI) score was found inversely related to the severity of COVID-19, and it significantly decreased in patients with common to severe forms of COVID-19 regardless of sex, age range, and body mass index (BMI) [
16].
Lack of proper pharmacological treatment for COVID-19 has encouraged alternative therapies to reduce the symptoms or severity of COVID-19 [
17]. Several observational studies have highlighted the association of nutrients with the altered immune response and increased severity and mortality of COVID-19 [
15,
18,
19]. Evidence-based studies suggest that nutritional status, including the level of micronutrients, plays an important role in the progression of COVID-19, where the selenium status of the body is ineludible [
18].
Selenium is an indispensable trace element required for the body’s normal functioning that imparts its physiological role via selenium-containing proteins called selenoproteins. Selenium is involved in innate and acquired immunity, including T and B lymphocyte function, and is necessary for antibody production and thus influences susceptibility to infections [
19]. The primary source of selenium is from the diet, which in turn depends on the soil selenium content and its bioavailability to crops [
20]. Numerous reports explain the beneficial effects of selenium in several human ailments such as cardiovascular diseases, obesity, diabetes, cancer, and microbial infections [
21].
3. Mechanism of SAR-CoV-2 Infection
Coronaviruses belong to crown-shaped animal viruses that mainly affect the respiratory system. The symptoms vary from the common cold to severe respiratory diseases. The Middle East Respiratory Syndrome virus (MERS-CoV) and severe acute respiratory syndrome virus (SARS-CoV) were the most recent infections caused by coronaviruses before COVID-19. Several canine and feline coronaviruses are present in animals, but they are harmless to humans, as they are not transmitted from animals to human beings. However, in rare cases, coronaviruses start to infect humans similar to what happened in the case of COVID-19, caused by a novel coronavirus, SARS-CoV-2 [
22].
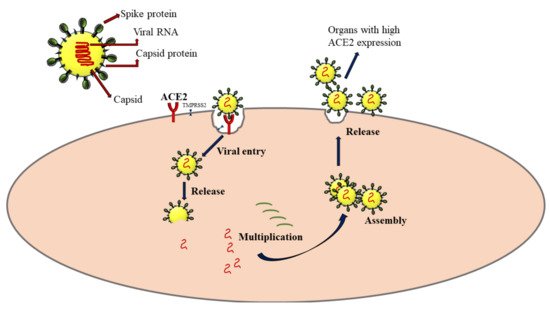
In humans, SAR-CoV-2 primarily affects the respiratory system, especially the alveolar epithelial cells in lung sacs. The crown-like spike proteins on the surface of viruses help them complex with the receptors such as angiotensin-converting enzyme 2 (ACE2) [
23]. After its entry into the host cell, it utilizes the host cellular machinery for synthesizing its genome materials and proteins. The virus progeny is released, which can infect new cells to propagate the infection (
Figure 2). The extent of cellular damage is a balance between host immunity and the ability of the virus to escape this defense. The severity of COVID-19 varies widely from asymptomatic and mildly symptomatic to deadly, critically depending on several pathophysiological conditions of the host. The ACE2 is widely expressed in lung, heart, kidney, neuronal, gastrointestinal, and liver cells [
24]. Severe SARS-CoV-2 infection has the potential to intrude on these tissues and damage these organs, leading to multiple organ damage [
25]. It is now proven that COVID-19 infection dysregulates cytokine homeostasis, leading to cytokine storm, characterized by severe systemic elevation of several pro-inflammatory cytokines. A cytokine storm can be edematous, causing acute respiratory distress syndrome (ARDS), pneumonia, and multiple organ failure in some patients [
26].
Figure 2. Mechanism of SARS-CoV-2 infection via the interaction with angiotensin-converting enzyme 2 (ACE2) receptors. TMPRSS2: Transmembrane protease, serine 2. The arrows represent the sequence of events following SARS-CoV-2 infection.
4. Selenoproteins in SAR-CoV-2 Infection
Proteins with the 21st amino acid, L-selenocysteine (Sec), are known as selenoproteins. Selenocysteine is encoded by the genetic codon UGA, which is otherwise a stop codon [
27]. During translation, Sec is co-translationally incorporated into a growing polypeptide chain with the assistance of unique elements such as Sec insertion sequence (SECIS), Sec-specific elongation factor (eEFsec), its own transfer RNA (tRNA[Ser]
Sec), the specific binding proteins for Sec insertion (SBP2), ribosomal protein L30, and nucleolin, to ensure the correct insertion of Sec into selenoproteins [
28,
29]. Around 25 selenoprotein genes have been characterized in humans, and some of these proteins play a pivotal role in maintaining redox homeostasis, modulating immune response and regulating inflammatory cascade [
30].
4.1. Glutathione Peroxidases (GPxs)
Glutathione peroxidases are involved in oxidative homeostasis and play a crucial role in viral pathogenesis. GPx1-4 and 6 are the five GPx isoforms that contain Sec in humans. GPxs catalyze the reduction of hydrogen peroxides using glutathione (GSH) as a cofactor to prevent oxidative stress. [
31]. They also regulate the redox signaling pathways involved in cell proliferation, differentiation, apoptosis, cytokine expression, ultimately affecting the overall health. Gpx1 and GPx4 are the most abundant proteins, while GPx3 is a glycosylated protein secreted in the plasma, whose activity correlates with the selenium status of the organism. GPx2 is present in epithelial tissues, and GPx6 is mainly associated with embryonic tissue and olfactory cells [
30].
GPx1 is a predominantly cytosolic protein with antiviral properties. Gene knockout studies in mice have shown that the absence of GPx1 overwhelms oxidative stress, resulting in enhanced pathogenicity and virulence of viruses. Lack of Gpx1 led to viral mutation and induced myocarditis in knockout mice compared to the wild type [
32]. A selenium-deficient diet increased the mutagenicity and virulence of benign coxsackievirus B3, suggesting the importance of selenium in GPx1 activity. Both selenium deficiency and GPx1 knockout heightened inflammatory responses and more severe lung pathology in response to influenza A infection, further indicating that GPX1 participates in molecular mechanisms protecting against viral infections of the respiratory tract [
33]. Wang et al. (2020) reported that GPx1 activity is reduced during COVID-19, affecting the expression of other selenoproteins. Reduced GPx1 level concurrently increases intracellular ROS and membrane lipid peroxidation in experimental studies performed with Se-deficient Vero E6 cells infected with SARS-CoV-2. These results emphasized the significance of Se for its potential use against COVID-19 [
34]. The interaction between GPx1 and M
pro, the main protease necessary for viral replication, is currently gaining attention as a potential therapeutic agent for COVID-19. Ebselen, a selenium-containing GPx1 mimetic, has emerged as the strongest inhibitor of M
pro and a target of great interest for therapeutic interventions for COVID-19 [
35].
Obesity is an important risk factor exacerbating the severity of COVID-19, which is often associated with cardiovascular disorders and thrombotic complications in critically ill SARS-CoV-2 patients [
36]. Obesity was found to decrease the activity of selenoproteins such as GPx3 in adipose tissues, of which the supplementation of selenium could improve. Thus, selenium supplementation was an effective supportive therapy for preventing cardiovascular impairments in obese COVID-19 patients admitted to the intensive care unit [
37].
SARS-CoV-2 infection is known to increase the production of mitochondrial ROS, helping viral replication [
38]. Of the different GPx isoforms, only GPx4 is located in the mitochondria, suggesting that targeting GPX4 increase may be beneficial. Currently, different mechanisms are being proposed for the role of GPx4 in COVID-19. Ferroptosis is a mechanism of programmed cell death caused by lipid-reactive oxygen species accumulation due to ferric ion overload in cells resulting in lipid peroxidation [
39]. Inactivation of GPx4 and glutathione depletion are considered as the major leading causes of ferroptosis, as GPx4 protects the cell membrane against lipid peroxidation and ferroptosis by eliminating lipid ROS [
40,
41]. GPx4 specifically protects the cells of the immune system against ferroptosis cell death. It is also proposed that leukopenia in COVID-19 patients may be associated with SARS-CoV-2-induced ferroptosis [
42]. Further, GPx4 is also essential for activating the stimulator of interferon genes, which is pivotal in initiating innate immune responses against viral infection [
42]. The findings also emphasize the importance of selenium compounds in the prophylaxis and treatment of COVID-19.
Infections of the olfactory epithelium result in a loss of smell in COVID-19 patients, one of the characteristic symptoms of SARS-CoV-2 infection. GPx6 is localized to the olfactory epithelium and is involved in scavenging hydrogen peroxide and hydroperoxides in humans and mice [
29]. It is proposed that Se supplementation could reduce olfactory mucosal inflammation, preventing viral infection/replication, including SARS-CoV-2.
4.2. Thioredoxin Reductases (TXNRDs)
Thioredoxin reductases (TXNRDs) are a family of selenoproteins that reduce thioredoxins and other endogenous and exogenous substrates, thereby controlling the expression of genes involved in cell growth, proliferation, and inflammation. Thioredoxins are necessary for DNA synthesis, as it converts ribonucleotides to deoxyribonucleotides. They are synthesized in response to oxidative stress and are upregulated by the nuclear factor-erythroid 2 related factor 2(NRF2), a cytoprotective transcription factor. Thioredoxins reduce oxidative stress and prevent viral propagation [
42]. Experimental evidence indicates that selenium-deficient Vero E6 cells infected with SARS-CoV-2 significantly downregulated TXNRD3 [
34].
4.3. Selenoprotein P (SELENOP)
SELENOP is one of the major circulatory antioxidant selenoproteins in plasma that helps to transport selenium to specific tissues. Moghaddam et al. (2020) observed lowered serum selenium and SELENOP concentrations in COVID-19 patients in Germany. The patients who died showed a significantly greater deficiency of selenoproteins, including SELENOP, than those who survived [
43]. Severe hypoxia and an increase in IL6, the hallmark of SARS-CoV-2 infection, are the other factors that suppress hepatic SELENOP expression, reducing circulating SELENOP levels and selenium delivery to tissues [
44,
45]. It was suggested that reducing the deficit of selenium and SELENOP in SARS-CoV-2-infected patients through supplementation could enable enhanced redox control and response to the immune system in COVID-19 management.
4.4. Other Selenoproteins
Recently, infection of selenium-deficient Vero E6 cells with SARS-CoV-2 revealed a downregulation of SELENOF, SELENOK, SELENOM, and SELENOS localized in the ER, indicating that infection could adversely affect ER [
34]. Compromised SELENOF and SELENOM proteins increase protein misfolding, while ER-associated degradation of misfolded proteins is attenuated by impaired SELENOK and SELENOS expression [
29]. Wang et al. found a profound reduction of SELENOF, which is targeted for proteolysis by the SARS-CoV-2 main protease M
pro [
46]. A large cohort study found a significantly higher risk of COVID-19-related death in African American ethnicity, which also harbors a polymorphic site at nucleotide position 1125 (A/G) located in the SECIS element of SELENOF, affecting the efficiency of selenocysteine incorporation into SELENOF in a Se-dependent fashion [
47,
48].
5. Role of Selenium on the Management of Viral Infection
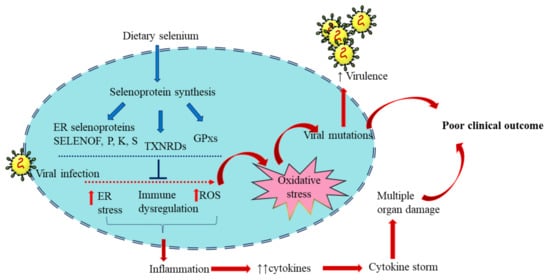
Selenium plays a vital role in managing viral infections through antioxidant, inflammatory, and immunomodulatory mechanisms to reduce the adversity of clinical manifestations (Figure 3).
Figure 3. Selenoproteins in viral infection: Dietary selenium is incorporated in selenoproteins that play regulatory roles in immune function and redox homeostasis. These include the Selenoproteins glutathione peroxidases (GPX), 15 kDa selenoprotein F (SELENOF), selenoproteins K and S and thioredoxin reductases (TXNRD). Viral infection alters the expression of selenoproteins and induces oxidative stress, increasing virus virulence. The resultant inflammation and excess cytokine production eventually lead to poor clinical outcomes. The blue arrows represent the sequence of events of selenoprotein synthesis from dietary selenium. The red arrows represent the consequence of viral infection. Adequate selenium levels can reduce the impact of viral infection and help in an optimum antiviral response.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23094809