There is a shortage of suitable tissue-engineered solutions for gingival recession, a soft tissue defect of the oral cavity. Autologous tissue grafts lead to an increase in morbidity due to complications at the donor site. Although material substitutes are available on the market, their development is early, and work to produce more functional material substitutes is underway. The latter materials along with newly conceived tissue-engineered substitutes must maintain volumetric form over time and have advantageous mechanical and biological characteristics facilitating the regeneration of functional gingival tissue.
- electrospinning
- gingival tissue
- material substitutes
1. Introduction
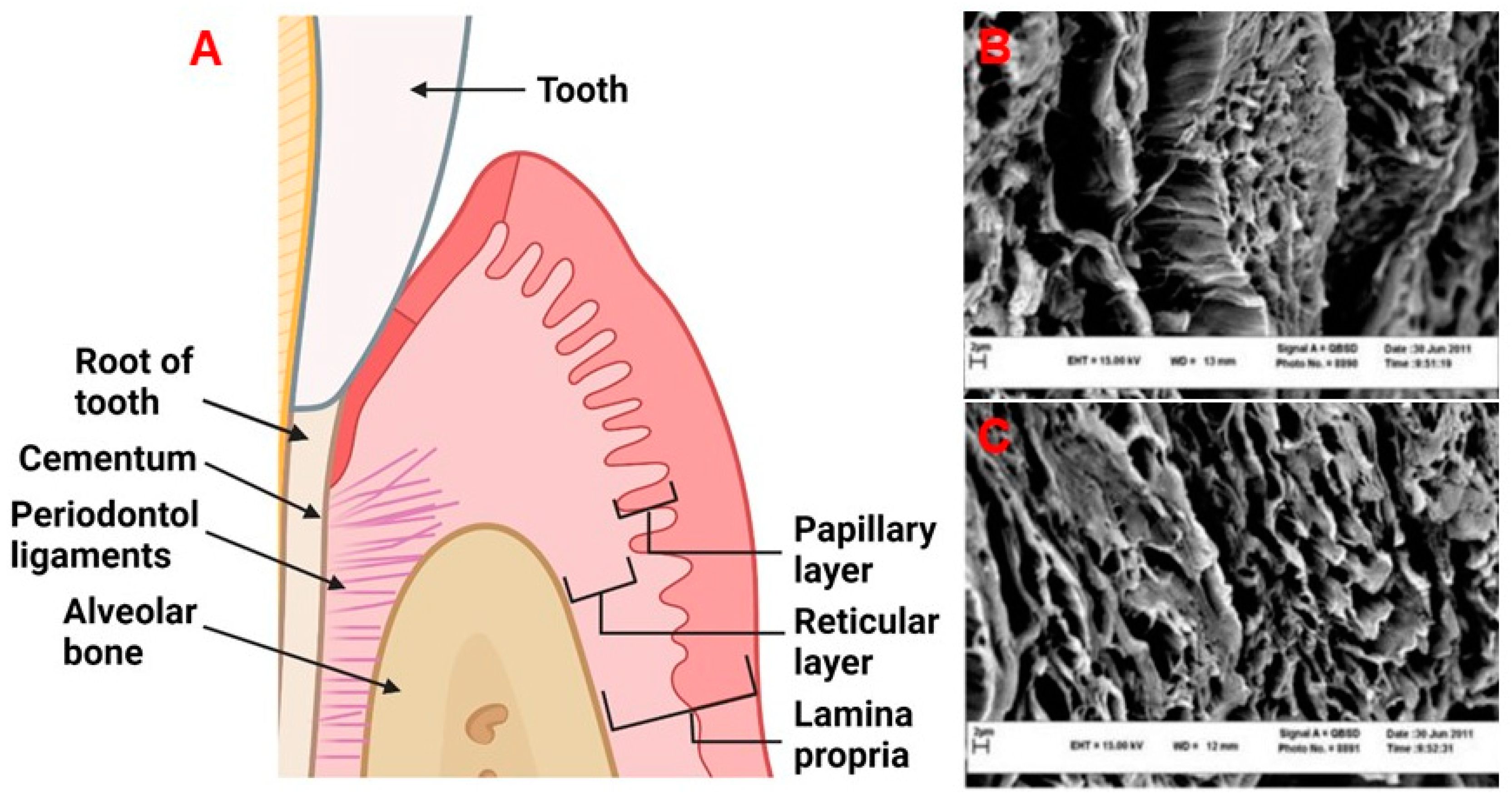
2. Physiology and Disease of the Periodontium and Gingival Tissues: Defining Structure Requirements
| Tissue Area | Type of Vessel | Diameter (µm) | Average Depth (µm) |
|---|---|---|---|
| Free gingiva | Capillary loops | ≤30 | 50–200 |
| Connective vessels | 50–100 | 200–700 | |
| Large blood vessels | 200–400 | ≥500 | |
| Attached gingiva | Capillary loops | ≤15 | 50–200 |
| Connective vessels | - | - | |
| Large blood vessels | 200–500 | ≥600 | |
| Alveolar mucosa | Capillary loops | ≤15 | 50–200 |
| Connective vessels | 200–600 | 200–700 | |
| Large blood vessels | ≥600 | ≥700 |
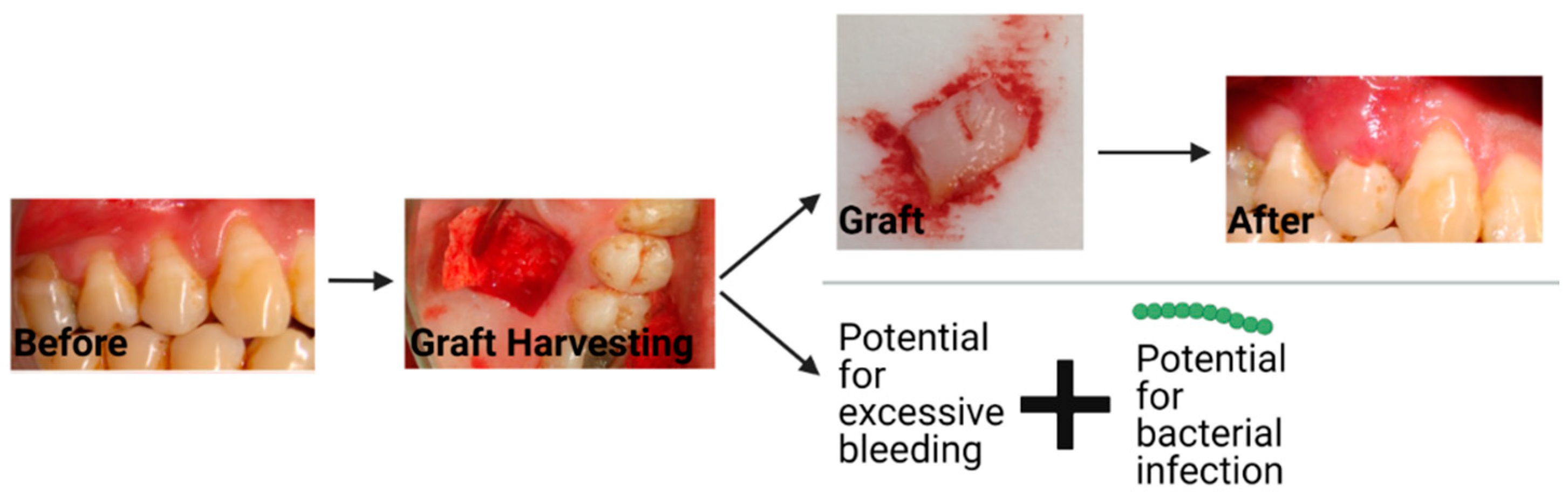
3. Current Material Options for Gingival Recession Treatment
This entry is adapted from the peer-reviewed paper 10.3390/ijms23095256
References
- Kassab, M.M.; Cohen, R.E. The etiology and prevalence of gingival recession. J. Am. Dent. Assoc. 2003, 134, 220–225.
- Eke, P.I.; Dye, B.A.; Wei, L.; Thronton-Evans, G.O.; Genco, R.J. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920.
- Merijohn, G.K. Management and prevention of gingival recession. Periodontol. 2000 2016, 71, 228–242.
- Moharamzadeh, K.; Colley, H.; Murdoch, C.; Hearnden, V.; Chai, W.L.; Brook, I.M.; Thornhill, M.H.; MacNeil, S. Tissue-engineered oral mucosa. J. Dent. Res. 2012, 91, 642–650.
- Mancini, L.; Fratini, A.; Tarallo, F.; Americo, L.M.; Marchetti, E. 3D analysis at implant sites after soft tissue augmentation with two types of collagen matrices: A pilot study. Plast. Aesthetic Res. 2021, 8, 26.
- Kim, D.M.; Neiva, R. Periodontal Soft Tissue Non–Root Coverage Procedures: A Systematic Review From the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S56–S72.
- Goudouri, O.M.; Kontonasaki, E.; Boccaccini, A.R. Layered Scaffolds for Periodontal Regeneration; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081009673.
- Benatti, B.B.; Silvério, K.G.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H., Jr. Physiological features of periodontal regeneration and approaches for periodontal tissue engineering utilizing periodontal ligament cells. J. Biosci. Bioeng. 2007, 103, 1–6.
- Herford, A.S.; Akin, L.; Cicciu, M.; Maiorana, C.; Boyne, P.J. Use of a Porcine Collagen Matrix as an Alternative to Autogenous Tissue for Grafting Oral Soft Tissue Defects. J. Oral Maxillofac. Surg. 2010, 68, 1463–1470.
- Zhang, J.; Wang, L.; Zhu, M.; Wang, L.; Xiao, N.; Kong, D. Wet-spun poly (ε-caprolactone) microfiber scaffolds for oriented growth and infiltration of smooth muscle cells. Mater. Lett. 2014, 132, 59–62.
- Smirani, R.; Rémy, M.; Devillard, R.; Naveau, A. Engineered Prevascularization for Oral Tissue Grafting: A Systematic Review. Tissue Eng. Part B Rev. 2020, 26, 383–398.
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721.
- Moharamzadeh, K.; Brook, I.M.; Van Noort, R.; Scutt, A.M.; Thornhill, M.H. Tissue-engineered oral mucosa: A review of the scientific literature. J. Dent. Res. 2007, 86, 115–124.
- Schroeder, H.E.; Listgarten, M.A. The gingival tissues: The architecture of periodontal protection. Periodontol. 2000 1997, 13, 91–120.
- Jati, A.S.; Furquim, L.Z.; Consolaro, A. Gingival recession: Its causes and types, and the importance of orthodontic treatment. Dental Press J. Orthod. 2016, 21, 18–29.
- Mahdavishahri, N.; Matin, M.M.; Fereidoni, M.; Yarjanli, Z.; Rad, S.A.B.; Ahmadi, S.K. In vitro assay of human gingival scaffold in differentiation of rat’s bone marrow mesenchymal stem cells to keratinocystes. Iran. J. Basic Med. Sci. 2012, 15, 1185–1190.
- Kaur, P.; Kakar, V. Collagen: Role in oral tissues: A review. Int. J. Sci. Res. 2014, 3, 273–276.
- Vindin, H.; Mithieux, S.M.; Weiss, A.S. Elastin architecture. Matrix Biol. 2019, 84, 4–16.
- Schoen, F.J.; Mitchell, R. Tissues, the Extracellular Matrix, and Cell Biomaterial Interactions, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Oxford, UK, 2013.
- Hughes, C.C.W. Endothelial-stromal interactions in angiogenesis. Curr. Opin. Hematol. 2008, 15, 204–209.
- Pitaru, S.; Melcher, A.H. Orientation of gingival fibroblasts and newly-synthesized collagen fibers in vitro: Resemblance to transseptal and dento-gingival fibers. J. Periodontal Res. 1983, 18, 483–500.
- Bullon, P.; Fioroni, M.; Goteri, G.; Rubini, C.; Battino, M. Immunohistochemical analysis of soft tissues in implants with healthy and peri-implantitis condition, and aggressive periodontitis. Clin. Oral Implant. Res. 2004, 15, 553–559.
- Yoshida, S.; Noguchi, K.; Imura, K.; Miwa, Y.; Sunohara, M.; Sato, I. A morphological study of the blood vessels associated with periodontal probing depth in human gingival tissue. Okajimas Folia Anat. Jpn. 2011, 88, 103–109.
- Le, N.M.; Song, S.; Zhou, H.; Xu, J.; Li, Y.; Sung, C.E.; Sadr, A.; Chung, K.H.; Subhash, H.M.; Kilpatrick, L.; et al. A noninvasive imaging and measurement using optical coherence tomography angiography for the assessment of gingiva: An in vivo study. J. Biophotonics 2018, 11, e201800242.
- Mikecs, B.; Vág, J.; Gerber, G.; Molnár, B.; Feigl, G.; Shahbazi, A. Revisiting the vascularity of the keratinized gingiva in the maxillary esthetic zone. BMC Oral Health 2021, 21, 160.
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984.
- Molnár, E.; Molnár, B.; Lohinai, Z.; Tóth, Z.; Benyó, Z.; Hricisák, L.; Windisch, P.; Vág, J. Evaluation of Laser Speckle Contrast Imaging for the Assessment of Oral Mucosal Blood Flow following Periodontal Plastic Surgery: An Exploratory Study. Biomed Res. Int. 2017, 2017, 4042902.
- Patel, M.; Nixon, P.J.; Chan, M.F.W. Gingival recession: Part 3. Surgical management using free grafts and guided tissue regeneration. Nat. Publ. Gr. 2011, 211, 353–358.
- Schmitt, C.M.; Tudor, C.; Kiener, K.; Wehrhan, F.; Schmitt, J.; Eitner, S.; Agaimy, A.; Schlegel, K.A. Vestibuloplasty: Porcine Collagen Matrix Versus Free Gingival Graft: A Clinical and Histologic Study. J. Periodontol. 2013, 84, 914–923.
- Bassetti, R.G.; Stähli, A.; Bassetti, M.A.; Sculean, A. Soft tissue augmentation around osseointegrated and uncovered dental implants: A systematic review. Clin. Oral Investig. 2017, 21, 53–70.
- Trivedi, S.R.; Bhavsar, N.V.; Dulani, K.; Trivedi, R. Clinical evaluation of subepithelial connective tissue graft and guided tissue regeneration for treatment of Miller’s class 1 gingival recession (comparative, split mouth, six months study). J. Clin. Exp. Dent. 2014, 6, 218–224.
- Dragan, I.F.; Hotlzman, L.P.; Karimbux, N.Y.; Morin, R.A.; Bassir, S.H. Clinical Outcomes of Comparing Soft Tissue Alternatives to Free Gingival Graft: A Systematic Review and Meta-Analysis. J. Evid. Based Dent. Pract. 2017, 17, 370–380.
- Smith, P.C.; Martínez, C.; Martínez, J.; Mcculloch, C.A. Role of fibroblast populations in periodontal wound healing and tissue remodeling. Front. Physiol. 2019, 10, 270.
- Sudbeck, B.D.; Pilcher, B.K.; Welgus, H.G.; Parks, W.C. Induction and repression of collagenase-1 by keratinocytes is controlled by distinct components of different extracellular matrix compartments. J. Biol. Chem. 1997, 272, 22103–22110.
- Zhang, C.; Lim, J.; Liu, J.; Ponugoti, B.; Alsadun, S.; Tian, C.; Vafa, R.; Graves, D.T. FOXO1 expression in keratinocytes promotes connective tissue healing. Sci. Rep. 2017, 7, 42834.
- Dugina, V.; Fontao, L.; Chaponnier, C.; Vasiliev, J.; Gabbiani, G. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J. Cell Sci. 2001, 114, 3285–3296.
- Levy, L.; Broad, S.; Diekmann, D.; Evans, R.D.; Watt, F.M. β1 Integrins regulate keratinocyte adhesion and differentiation by distinct mechanisms. Mol. Biol. Cell 2000, 11, 453–466.
- Groeger, S.E.; Meyle, J. Epithelial barrier and oral bacterial infection. Periodontol. 2000 2015, 69, 46–67.
- Narayan Biswal, B.; Narayan Das, S.; Kumar Das, B.; Rath, R. Alteration of cellular metabolism in cancer cells and its therapeutic. J. Oral Maxillofac. Pathol. 2017, 21, 244–251.
- Yoshizawa, M.; Koyama, T.; Kojima, T.; Kato, H.; Ono, Y.; Saito, C. Keratinocytes of tissue-engineered human oral mucosa promote re-epithelialization after intraoral grafting in athymic mice. J. Oral Maxillofac. Surg. 2012, 70, 1199–1214.
- Izumi, K.; Feinberg, S.E.; Terashi, H.; Marcelo, C.L. Evaluation of transplanted tissue-engineered oral mucosa equivalents in severe combined immunodeficient mice. Tissue Eng. 2003, 9, 163–174.
- McGuire, M.K.; Scheyer, E.T. Randomized, Controlled Clinical Trial to Evaluate a Xenogeneic Collagen Matrix as an Alternative to Free Gingival Grafting for Oral Soft Tissue Augmentation. J. Periodontol. 2014, 85, 1333–1341.
- Vallecillo, C.; Toledano-Osorio, M.; Vallecillo-Rivas, M.; Toledano, M.; Osorio, R. In Vitro biodegradation pattern of collagen matrices for soft tissue augmentation. Polymers 2021, 13, 2633.
- Caballé-Serrano, J.; Zhang, S.; Ferrantino, L.; Simion, M.; Chappuis, V.; Bosshardt, D.D. Tissue response to a porous collagen matrix used for soft tissue augmentation. Materials 2019, 12, 3721.
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.L.; Giannobile, W.V. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J. Periodontol. 2020, 91, 17–25.
- Wei, P.-C.; Laurell, L.; Geivelis, M.; Lingen, M.W.; Maddalozzo, D.; Increased, A.; Gingiva, A. Acellular Dermal Matrix Allografts to Achieve Increased Attached Gingiva. Part 1. A Clinical Study. J. Periodontol. 2000, 71, 1297–1305.
- Romeu, D.; De Resende, B.; Luiz, S.; Greghi, A.; Siqueira, A.F.; Augusto, C.; Benfatti, M.; Damante, C.A.; Schutzer, M.; Zangrando, R.; et al. Acellular dermal matrix allograft versus free gingival graft: A histological evaluation and split-mouth randomized clinical trial. Clin. Oral Investig. 2019, 23, 539–550.
