The chronicity of wounds is affected by several contributory factors, including hormonal imbalances, cytokines, invasive microbial infections, and growth factors. More importantly, bacterial infections have been implicated as the predominant feature in most chronic wound microenvironments, including Staphylococcus aureus and Pseudomonas aeruginosa. These bacteria exist in polymicrobial forms forming biofilms that afford them protection against the host’s immunity and conventional antibiotics. S. aureus biofilms are sometimes present close to the surface of chronic wounds, while P. aeruginosa biofilms appear deep within wound tissue. The recalcitrant disposition of these microbes has been implicated as one of the causalities of antimicrobial resistance.
- antimicrobial
- anti-inflammatory
- antibiotics-free
- tissue regeneration
- chronic wound healing
1. Wound Healing Process
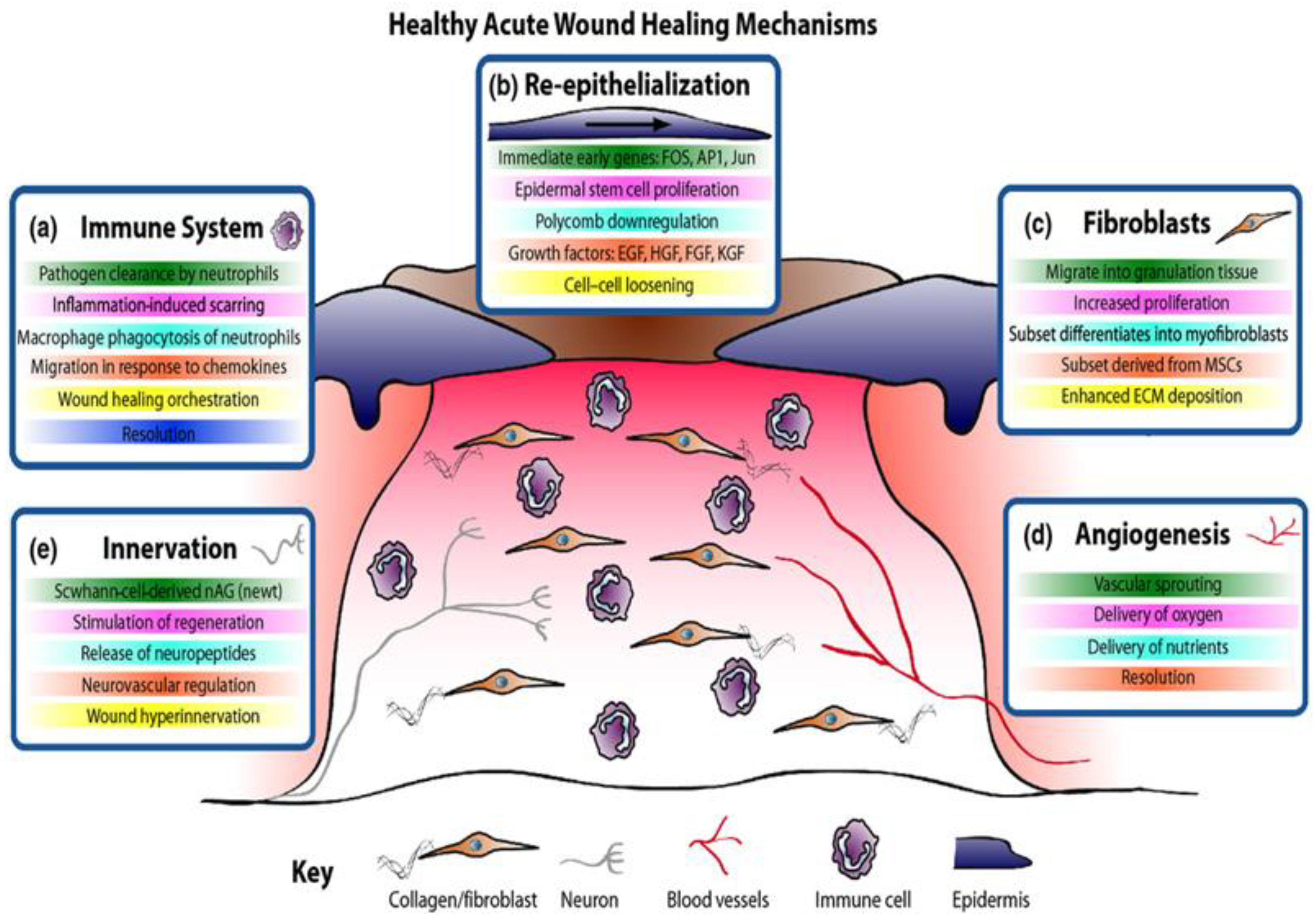
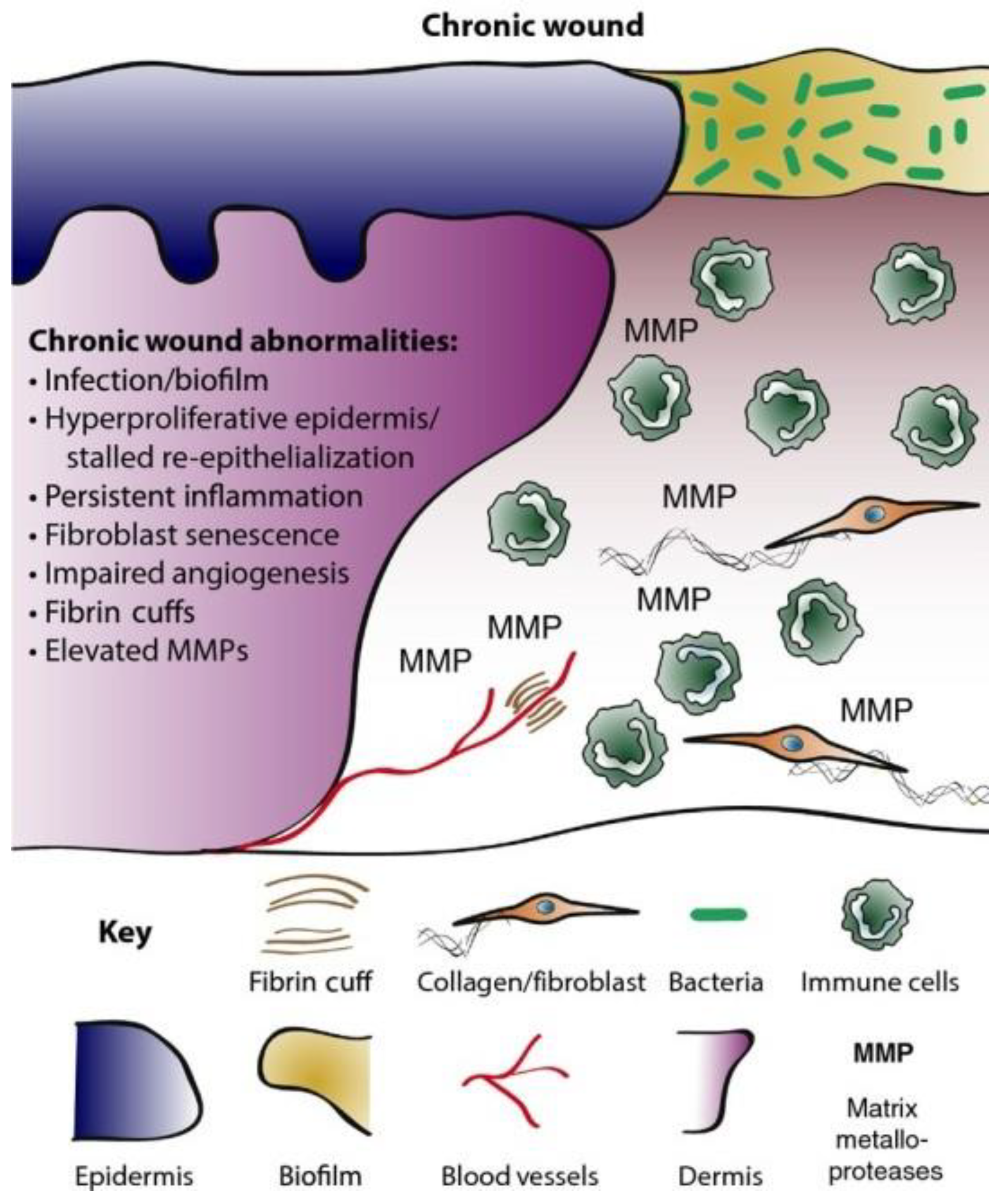
2. Chronic Wounds
3. Antimicrobial Resistance (AMR) in Chronic Wounds
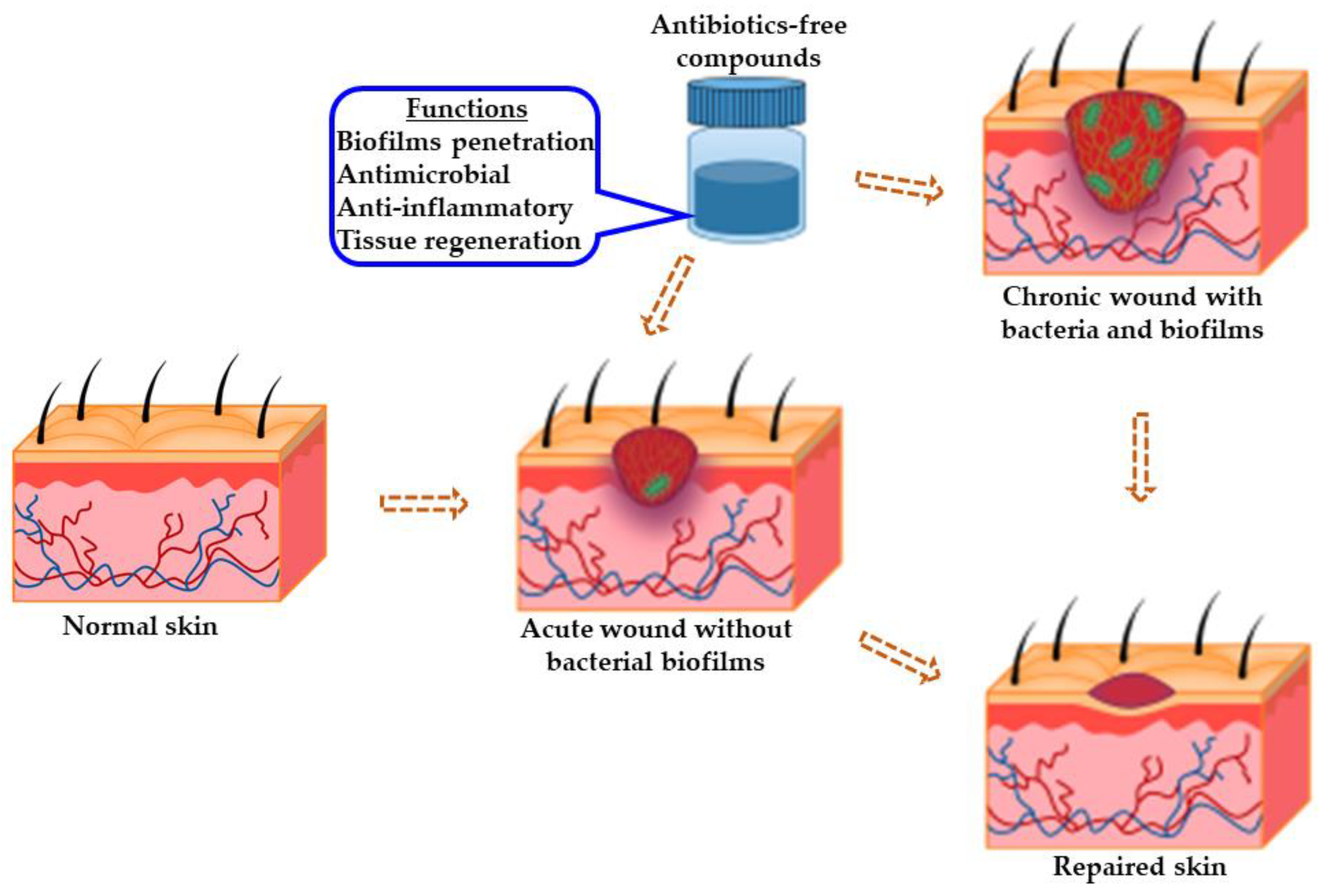
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14051021
References
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7.
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378.
- Enoch, S.; Grey, J.E.; Harding, K. Recent advances and emerging treatments. BMJ 2006, 332, 962–965.
- Topman, G.; Lin, F.-H.; Gefen, A. The natural medications for wound healing—Curcumin, Aloe-Vera and Ginger—do not induce a significant effect on the migration kinematics of cultured fibroblasts. J. Biomech. 2013, 46, 170–174.
- Kulac, M.; Aktas, C.; Tulubas, F.; Uygur, R.; Kanter, M.; Erboga, M.; Ceber, M.; Topcu, B.; Ozen, O.A. The effects of topical treatment with curcumin on burn wound healing in rats. J. Mol. Histol. 2012, 44, 83–90.
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97 (Pt B), 417–426.
- Kaiser, P.; Wächter, J.; Windbergs, M. Therapy of infected wounds: Overcoming clinical challenges by advanced drug delivery systems. Drug Deliv. Transl. Res. 2021, 11, 1545–1567.
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014, 22, 174–186.
- James, G.A.; Swogger, E.; Wolcott, R.; deLancey Pulcini, E.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in chronic wounds. Wound Repair Regen. 2008, 16, 37–44.
- Schierle, C.F.; De La Garza, M.; Mustoe, T.A.; Galiano, R.D. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009, 17, 354–359.
- Gray, D.; Barrett, S.; Battacharyya, M.; Butcher, M.; Enoch, S.; Fumerola, S.; Stephen-Haynes, J.; Edwards-Jones, V.; Leaper, D.; Strohal, R.; et al. PHMB and its potential contribution to wound management. Wounds 2010, 6, 40–46.
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25.
- Kadam, S.; Nadkarni, S.; Lele, J.; Sakhalkar, S.; Mokashi, P.; Kaushik, K.S. Bioengineered Platforms for Chronic Wound Infection Studies: How Can We Make Them More Human-Relevant? Front. Bioeng. Biotechnol. 2019, 7, 418.
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10.
- Stewart, P.S. Antimicrobial Tolerance in Biofilms. Microbiol. Spectr. 2015, 3.
- Song, T.; Duperthuy, M.; Wai, S.N. Sub-Optimal Treatment of Bacterial Biofilms. Antibiotics 2016, 5, 23.
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301.
- Tolker-Nielsen, T. Biofilm Development. Microbiol. Spectr. 2015, 3.
- Zheng, Y.; Wang, D.; Ma, L. Effect of Polyhexamethylene Biguanide in Combination with Undecylenamidopropyl Betaine or PslG on Biofilm Clearance. Int. J. Mol. Sci. 2021, 22, 768.
- Topa, S.H.; Subramoni, S.; Palombo, E.A.; Kingshott, P.; Rice, S.A.; Blackall, L.L. Cinnamaldehyde disrupts biofilm formation and swarming motility of Pseudomonas aeruginosa. Microbiology 2018, 164, 1087–1097.
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403.
- Wolcott, R.D.; Rhoads, D.D.; Dowd, S.E. Biofilms and chronic wound inflammation. J. Wound Care 2008, 17, 333–341.
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6.
- Cooper, R.; Kirketerp-Møller, K. Non-antibiotic antimicrobial interventions and antimicrobial stewardship in wound care. J. Wound Care 2018, 27, 355–377.
- World Health Organization (WHO). New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis. 2019. Available online: https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 11 February 2022).
- Crofts, T.S.; Gasparrini, A.J.; Dantas, G. Next-generation approaches to understand and combat the antibiotic resistome. Nat. Rev. Microbiol. 2017, 15, 422–434.
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327.
- Ibrahim, N.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A.N. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Health 2018, 15, 2360.
- Mun, S.-H.; Joung, D.-K.; Kim, Y.-S.; Kang, O.-H.; Kim, S.-B.; Seo, Y.-S.; Kim, Y.-C.; Lee, D.-S.; Shin, D.-W.; Kweon, K.-T.; et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718.
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80.
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203.
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305.
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310.
- Hayek, S.A.; Gyawali, R.; Ibrahim, S.A. Antimicrobial Natural Products (A. Méndez-Vilas, Ed.), Microbial Pathogens and Strategies for Combating Them: Science, Technology, and Education © FORMATEX 2013, 910–921. 2013. Available online: https://tarjomefa.com/wp-content/uploads/2016/11/5690-English.pdf (accessed on 10 February 2022).
