The ETS-related gene (ERG) is proto-oncogene that is classified as a member of the ETS transcription factor family, which has been found to be consistently overexpressed in about half of the patients with clinically significant prostate cancer (PCa). The overexpression of ERG can mostly be attributed to the fusion of the ERG and transmembrane serine protease 2 (TMPRSS2) genes, and this fusion is represented about 85% of all gene fusions observed in prostate cancer. Clinically, individuals with ERG gene fusion are mostly documented to have advanced tumor stages, increased mortality, and higher rates of metastasis in non-surgical cohorts.
- gene fusion
- prostate cancer
- tumorigenesis
1. Structural Characteristics and Allosteric Autoinhibition of ERG
2. The Function of ETS-Related Gene (ERG) in Normal Cell Types
3. Prominent TMPRSS2-ERG Gene Fusion Found in PCa
4. Functional ERG Overexpression in Prostate Cancer Cells
5. TMPRSS2-ERG Fusion Upregulates CXCR4 Enhances Tumor Adhesion and Aggregation
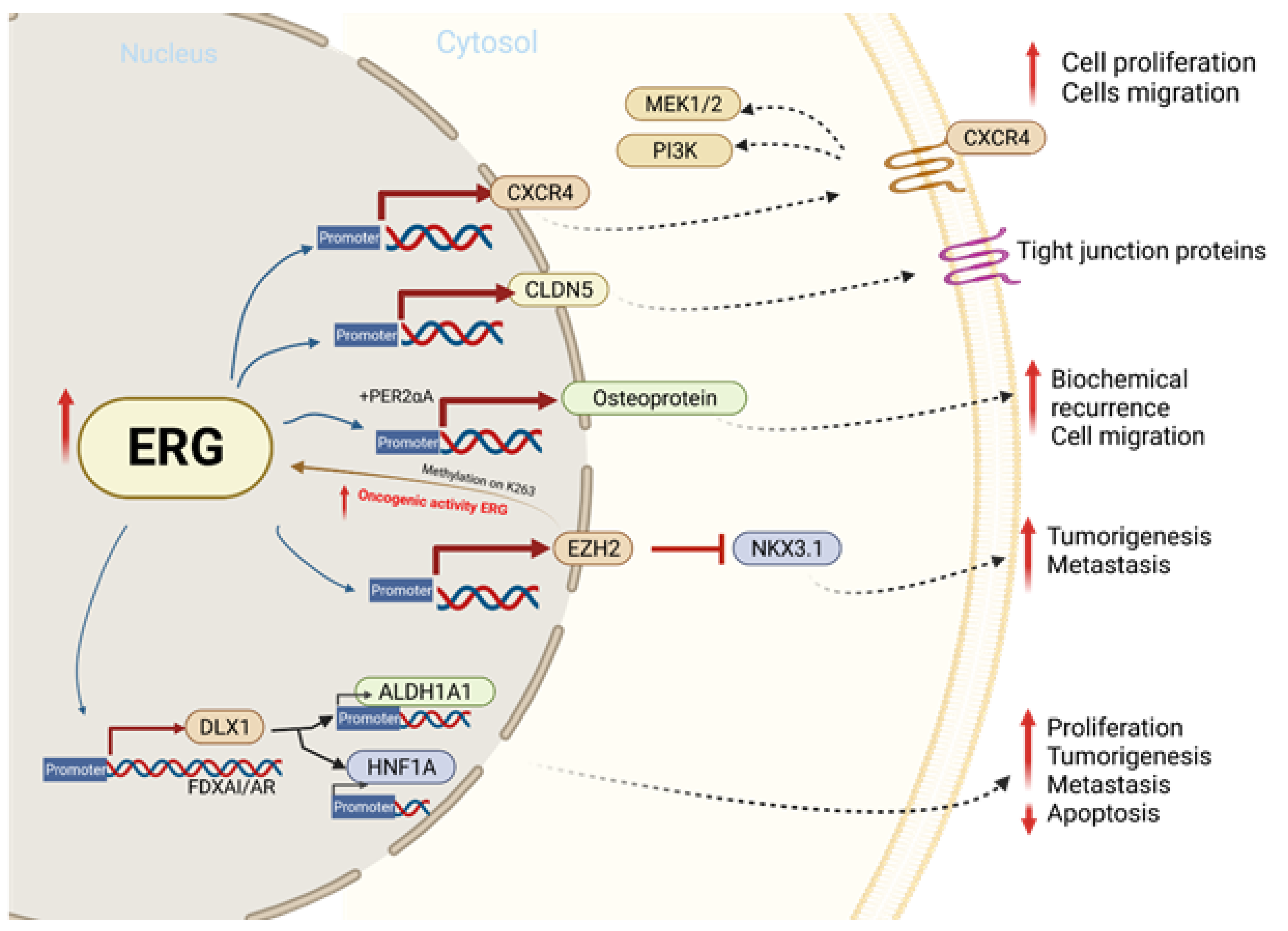
6. TMPRSS2-ERG Binds to EMT Key Regulators ZEB1/ZEB2
7. EZH2 Enhances ERG Oncogenic Activity
8. ERG and Tight Junction Protein CLDN5
9. ERG Upregulates Distal-Less Homeobox-1(DLX1)
10. GSK3B and WEE1 Induces TMPRSS2-ERG Degradation
This entry is adapted from the peer-reviewed paper 10.3390/ijms23094772
References
- Adamo, P.; Ladomery, M.R. The oncogene ERG: A key factor in prostate cancer. Oncogene 2016, 35, 403–414.
- Babal, Y.; Kandemir, B.; Kurnaz, I. Gene Regulatory Network of ETS Domain Transcription Factors in Different Stages of Glioma. J. Pers. Med. 2021, 11, 138.
- Parolia, A.; Cieslik, M.; Chu, S.-C.; Xiao, L.; Ouchi, T.; Zhang, Y.; Wang, X.; Vats, P.; Cao, X.; Pitchiaya, S.; et al. Distinct structural classes of activating FOXA1 alterations in advanced prostate cancer. Nature 2019, 571, 413–418.
- Majesky, M.W. Vascular Development. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e17–e24.
- Vivekanand, P. Lessons from Drosophila Pointed, an ETS family transcription factor and key nuclear effector of the RTK signaling pathway. Genesis 2018, 56, e23257.
- Pham, D.; Moseley, C.E.; Gao, M.; Savic, D.; Winstead, C.J.; Sun, M.; Kee, B.L.; Myers, R.M.; Weaver, C.T.; Hatton, R.D. Batf Pioneers the Reorganization of Chromatin in Developing Effector T Cells via Ets1-Dependent Recruitment of Ctcf. Cell Rep. 2019, 29, 1203–1220.e7.
- Vasuri, F.; Valente, S.; Motta, I.; Degiovanni, A.; Ciavarella, C.; Pasquinelli, G. ETS-Related Gene Expression in Healthy Femoral Arteries With Focal Calcifications. Front. Cell Dev. Biol. 2021, 9, 623782.
- Tharakan, B.; Hunter, F.A.; Muthusamy, S.; Randolph, S.; Byrd, C.; Rao, V.N.; Reddy, E.S.P.; Childs, E.W. ETS-Related Gene Activation Preserves Adherens Junctions and Permeability in Microvascular Endothelial Cells. Shock 2021, 57, 309–315.
- Neal, A.; Nornes, S.; Louphrasitthiphol, P.; Sacilotto, N.; Preston, M.D.; Fleisinger, L.; Payne, S.; De Val, S. ETS factors are required but not sufficient for specific patterns of enhancer activity in different endothelial subtypes. Dev. Biol. 2021, 473, 1–14.
- Looney, A.P.; Han, R.; Stawski, L.; Marden, G.; Iwamoto, M.; Trojanowska, M. Synergistic Role of Endothelial ERG and FLI1 in Mediating Pulmonary Vascular Homeostasis. Am. J. Respir. Cell Mol. Biol. 2017, 57, 121–131.
- Lorenzin, F.; Demichelis, F. Past, Current, and Future Strategies to Target ERG Fusion-Positive Prostate Cancer. Cancers 2022, 14, 1118.
- Kohaar, I.; Li, Q.; Chen, Y.; Ravindranath, L.; Young, D.; Ali, A.; Sesterhenn, I.A.; Rosner, I.L.; Cullen, J.; Srivastava, S.; et al. Association of germline genetic variants with TMPRSS2-ERG fusion status in prostate cancer. Oncotarget 2020, 11, 1321–1333.
- Kobelyatskaya, A.; Pudova, E.; Snezhkina, A.; Fedorova, M.; Pavlov, V.; Guvatova, Z.; Savvateeva, M.; Melnikova, N.; Dmitriev, A.; Trofimov, D.; et al. Impact TMPRSS2–ERG Molecular Subtype on Prostate Cancer Recurrence. Life 2021, 11, 588.
- Giunchi, F.; Massari, F.; Altimari, A.; Gruppioni, E.; Nobili, E.; Fiorentino, M.; Ardizzoni, A. Dual TMPRSS2:ERG Fusion in a Patient with Lung and Prostate Cancers. Diagnostics 2020, 10, 1109.
- Yamoah, K.; Lal, P.; Awasthi, S.; Naghavi, A.O.; Rounbehler, R.J.; Gerke, T.; Berglund, A.E.; Pow-Sang, J.M.; Schaeffer, E.M.; Dhillon, J.; et al. TMPRSS2-ERG fusion impacts anterior tumor location in men with prostate cancer. Prostate 2020, 81, 109–117.
- Fang, L.; Li, D.; Yin, J.; Pan, H.; Ye, H.; Bowman, J.; Capaldo, B.; Kelly, K. TMPRSS2-ERG promotes the initiation of prostate cancer by suppressing oncogene-induced senescence. Cancer Gene Ther. 2022, 1–14.
- Sakamoto, K.; Endo, K.; Sakamoto, K.; Kayamori, K.; Ehata, S.; Ichikawa, J.; Ando, T.; Nakamura, R.; Kimura, Y.; Yoshizawa, K.; et al. EHF suppresses cancer progression by inhibiting ETS1-mediated ZEB expression. Oncogenesis 2021, 10, 26.
- Greulich, B.M.; Plotnik, J.P.; Jerde, T.J.; Hollenhorst, P.C. Toll-like receptor 4 signaling activates ERG function in prostate cancer and provides a therapeutic target. NAR Cancer 2021, 3, zcaa046.
- Tsourlakis, M.C.; Khosrawi, P.; Weigand, P.; Kluth, M.; Hube-Magg, C.; Minner, S.; Koop, C.; Graefen, M.; Heinzer, H.; Wittmer, C.; et al. VEGFR-1 Overexpression Identifies a Small Subgroup of Aggressive Prostate Cancers in Patients Treated by Prostatectomy. Int. J. Mol. Sci. 2015, 16, 8591–8606.
- Sun, C.; Dobi, A.; Mohamed, A.; Li, H.; Thangapazham, R.L.; Furusato, B.; Shaheduzzaman, S.; Tan, S.H.; Vaidyanathan, G.; Whitman, E.; et al. TMPRSS2-ERG fusion, a common genomic alteration in prostate cancer activates C-MYC and abrogates prostate epithelial differentiation. Oncogene 2008, 27, 5348–5353.
- Vestweber, D. VE-cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 223–232.
- Cai, J.; Kandagatla, P.; Singareddy, R.; Kropinski, A.; Sheng, S.; Cher, M.L.; Chinni, S.R. Androgens Induce Functional CXCR4 through ERG Factor Expression in TMPRSS2-ERG Fusion-Positive Prostate Cancer Cells. Transl. Oncol. 2010, 3, 195–203.
- Clézardin, P.; Coleman, R.; Puppo, M.; Ottewell, P.; Bonnelye, E.; Paycha, F.; Confavreux, C.B.; Holen, I. Bone metastasis: Mechanisms, therapies, and biomarkers. Physiol. Rev. 2021, 101, 797–855.
- Sun, Y.X.; Fang, M.; Wang, J.; Cooper, C.R.; Pienta, K.J.; Taichman, R.S. Expression and activation of alpha v beta 3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate 2007, 67, 61–73.
- Singareddy, R.; Semaan, L.; Conley-LaComb, M.K.; John, J.S.; Powell, K.; Iyer, M.; Smith, D.; Heilbrun, L.K.; Shi, N.; Sakr, W.; et al. Transcriptional Regulation of CXCR4 in Prostate Cancer: Significance of TMPRSS2-ERG Fusions. Mol. Cancer Res. 2013, 11, 1349–1361.
- Zarrabi, A.; Hashemi, F.; Hashemi, F.; Zabolian, A.; Banihashemi, S.M.; Moghadam, S.S.; Hushmandi, K.; Samarghandian, S.; Ashrafizadeh, M.; Khan, H. Role of ZEB Family Members in Proliferation, Metastasis, and Chemoresistance of Prostate Cancer Cells: Revealing Signaling Networks. Curr. Cancer Drug Targets 2021, 21, 749–767.
- Wang, J.; Farkas, C.; Benyoucef, A.; Carmichael, C.; Haigh, K.; Wong, N.; Huylebroeck, D.; Stemmler, M.P.; Brabletz, S.; Brabletz, T.; et al. Interplay between the EMT transcription factors ZEB1 and ZEB2 regulates hematopoietic stem and progenitor cell differentiation and hematopoietic lineage fidelity. PLOS Biol. 2021, 19, e3001394.
- Zoma, M.; Curti, L.; Shinde, D.; Albino, D.; Mitra, A.; Sgrignani, J.; Mapelli, S.N.; Sandrini, G.; Civenni, G.; Merulla, J.; et al. EZH2-induced lysine K362 methylation enhances TMPRSS2-ERG oncogenic activity in prostate cancer. Nat. Commun. 2021, 12, 4147.
- Margueron, R.; Reinberg, D. The Polycomb complex PRC2 and its mark in life. Nature 2011, 469, 343–349.
- Yuan, L.; Le Bras, A.; Sacharidou, A.; Itagaki, K.; Zhan, Y.; Kondo, M.; Carman, C.V.; Davis, G.E.; Aird, W.C.; Oettgen, P. ETS-related Gene (ERG) Controls Endothelial Cell Permeability via Transcriptional Regulation of the Claudin 5 (CLDN5) Gene. J. Biol. Chem. 2012, 287, 6582–6591.
- Liu, F.; Liu, Q.; Yuan, F.; Guo, S.; Liu, J.; Sun, Z.; Gao, P.; Wang, Y.; Yan, S.; Liu, J. Erg mediates downregulation of claudin-5 in the brain endothelium of a murine experimental model of cerebral malaria. FEBS Lett. 2019, 593, 2585–2595.
- Ma, S.-C.; Li, Q.; Peng, J.-Y.; Zhouwen, J.-L.; Diao, J.-F.; Niu, J.-X.; Wang, X.; Guan, X.-D.; Jia, W.; Jiang, W.-G. Claudin-5 regulates blood-brain barrier permeability by modifying brain microvascular endothelial cell proliferation, migration, and adhesion to prevent lung cancer metastasis. CNS Neurosci. Ther. 2017, 23, 947–960.
- Kind, S.; Büscheck, F.; Höflmayer, D.; Hube-Magg, C.; Kluth, M.; Tsourlakis, M.C.; Steurer, S.; Clauditz, T.S.; Luebke, A.M.; Burandt, E.; et al. Claudin-1 upregulation is associated with favorable tumor features and a reduced risk for biochemical recurrence in ERG-positive prostate cancer. World J. Urol. 2019, 38, 2185–2196.
- Tian, R.; Luo, Y.; Liu, Q.; Cai, M.; Li, J.; Sun, W.; Wang, J.; He, C.; Liu, Y.; Liu, X. The effect of claudin-5 overexpression on the interactions of claudin-1 and -2 and barrier function in retinal cells. Curr. Mol. Med. 2014, 14, 1226–1237.
- Kluger, M.S.; Clark, P.R.; Tellides, G.; Gerke, V.; Pober, J.S. Claudin-5 Controls Intercellular Barriers of Human Dermal Microvascular but Not Human Umbilical Vein Endothelial Cells. Arter. Thromb. Vasc. Biol. 2013, 33, 489–500.
- Goel, S.; Bhatia, V.; Kundu, S.; Biswas, T.; Carskadon, S.; Gupta, N.; Asim, M.; Morrissey, C.; Palanisamy, N.; Ateeq, B. Transcriptional network involving ERG and AR orchestrates Distal-less homeobox-1 mediated prostate cancer progression. Nat. Commun. 2021, 12, 5325.
- Cani, A.K.; Hu, K.; Liu, C.J.; Siddiqui, J.; Zheng, Y.; Han, S.; Nallandhighal, S.; Hovelson, D.H.; Xiao, L.; Pham, T.; et al. Development of a Whole-urine, Multiplexed, Next-generation RNA-sequencing Assay for Early Detection of Aggressive Prostate Cancer. Eur. Urol. Oncol. 2021.
- Van Neste, L.; Hendriks, R.J.; Dijkstra, S.; Trooskens, G.; Cornel, E.B.; Jannink, S.A.; de Jong, H.; Hessels, D.; Smit, F.P.; Melchers, W.J.G.; et al. Detection of High-grade Prostate Cancer Using a Urinary Molecular Biomarker–Based Risk Score. Eur. Urol. 2016, 70, 740–748.
- Hong, Z.; Zhang, W.; Ding, D.; Huang, Z.; Yan, Y.; Cao, W.; Pan, Y.; Hou, X.; Weroha, S.J.; Karnes, R.J.; et al. DNA Damage Promotes TMPRSS2-ERG Oncoprotein Destruction and Prostate Cancer Suppression via Signaling Converged by GSK3beta and WEE1. Mol. Cell 2020, 79, 1008–1023.
- Strittmatter, B.G.; Jerde, T.J.; Hollenhorst, P.C. Ras/ERK and PI3K/AKT signaling differentially regulate oncogenic ERG medi-ated transcription in prostate cells. PLoS Genet. 2021, 17, e1009708.
